43 Lewis Diagram For O2
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen)).For the O2 structure use the periodic table to find the t... Steps of drawing lewis structure of CO 2. There are several steps to draw the lewis structure of CO 2 and studying this carefully will guide you to draw lewis structures easily in your examinations.. Determine total number of electrons of the valance shells of carbon and oxygen atoms; Total electrons pairs existing as lone pairs and bonds
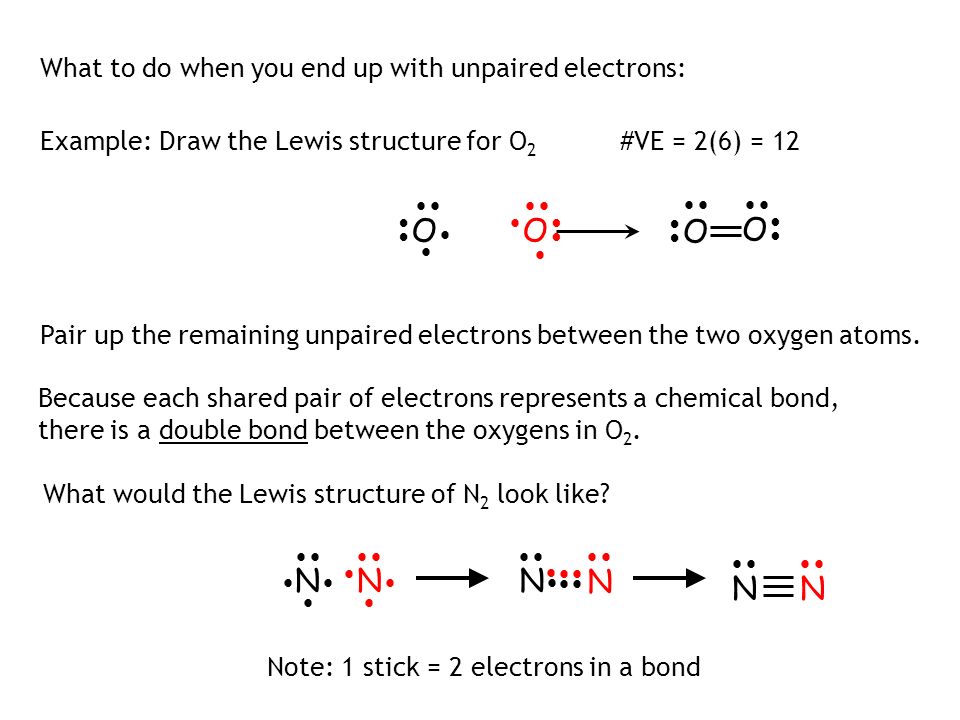
Lewis Structures for O2. Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar - electrons are shared equally. Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen.

Lewis diagram for o2
Lewis Structure for O2 (Dioxygen or Oxygen Gas) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. -the physical properties of a molecule such as boiling point, surface tension, etc. Lewis Dot Diagram For Oxygen. Oxygen has a special rule when doubling. Lewis dot diagrams displaying higher than optimal formal charges represent higher energy states of the species. Lewis dot structures for the first few non-‐metals. The lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared. O2 Properties. The O 2 Lewis structure shows two oxygen atoms bonded in the same way to each other. It’s perfectly symmetric. Generally, small symmetric molecules are nonpolar. The O 2 Lewis structure indicates that the O 2 molecule is perfectly symmetric. Therefore, O 2 is a nonpolar substance. Small nonpolar substances tend to be gasses.
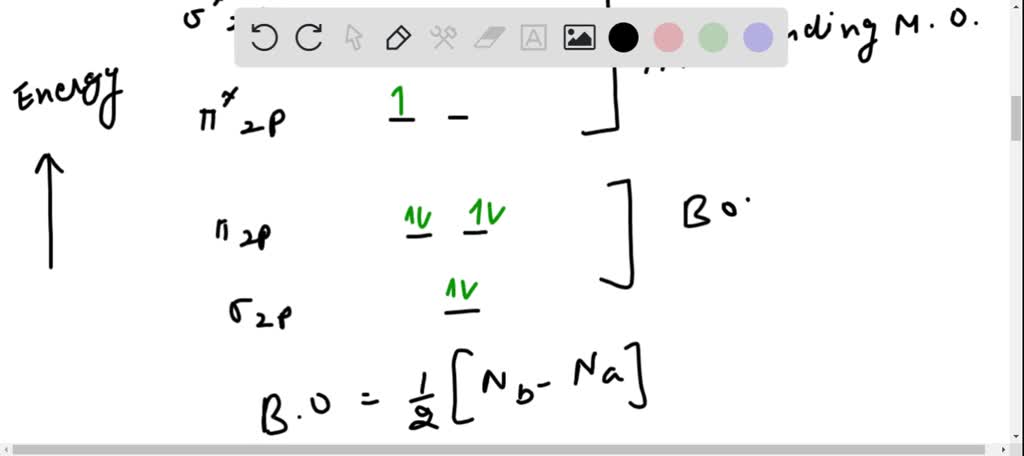
Lewis diagram for o2. Chemistry questions and answers. Lewis Dot Diagrams -- Diatomic Molecules and Ions Use the check-boxes to select all (and only) those electron dot pictures for the indicated molecules or ions which are acceptable Lewis structures. F::F* Lewis structure of F2? :N::N: Lewis structure of N2? :C::O: Lewis structure of CO? :0010: Lewis structure of O2? 1-Draw the Lewis- dot structure of O2. Can you predict the magnetic properties based on your diagram? How many unpaired electrons do you predict? 2-Draw the molecular orbitals for the following orbital overlap. a. Overlap of s and Pz orbitals. b. Overlap of Pz and Pz orbitals. c. Overlap of Px/Py with Px/Py orbitals. d. Overlap of dx2-y2 and Pz. e. O2 Properties. The O 2 Lewis structure shows two oxygen atoms bonded in the same way to each other. It’s perfectly symmetric. Generally, small symmetric molecules are nonpolar. The O 2 Lewis structure indicates that the O 2 molecule is perfectly symmetric. Therefore, O 2 is a nonpolar substance. Small nonpolar substances tend to be gasses. How to draw the Lewis Structure of Oxygen Gas - with explanation!Check me out: http://www.chemistnate
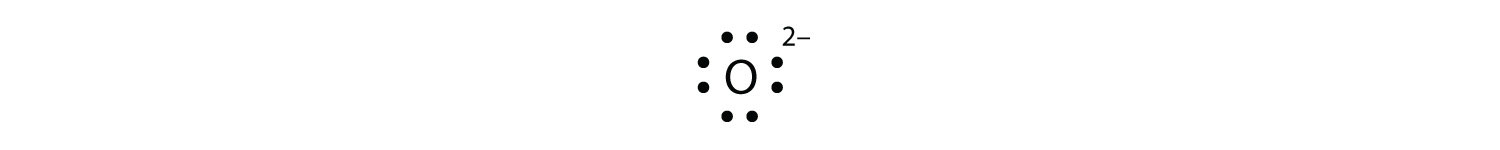
O 2 2-(peroxide ion) Lewis Structure. O 2 2-(peroxide ion) anion contains only two oxygen atoms. Peroxide anion has -2 charge. In O 2 2-lewis structure, each oxygen atom has -1 charge and three lone pairs. Both oxygen atoms are joint through a single bond. In this tutorial, we are going to draw the lewis structure of O 2 2-ion step by step. Lewis Dot Diagram For Oxygen. Oxygen has a special rule when doubling. Lewis dot diagrams displaying higher than optimal formal charges represent higher energy states of the species. Lewis dot structures for the first few non-‐metals. The lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared. Lewis Structure for O2 (Dioxygen or Oxygen Gas) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. -the physical properties of a molecule such as boiling point, surface tension, etc. Answer: Do you mean the peroxide anion? O2^-2? You don't have to write those negatives. That's just showing charge on each side and it was the best I found on Google. If you want to know methods of determining Lewis structures, I'll be glad to point you in the right direction or do examples with...
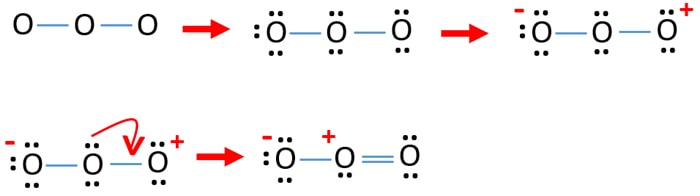
O2 is an allotrope of oxygen and is made out of two oxygen atoms bound together. Although the chemical formula for this allotrope is O2, it is frequently just referred to as oxygen. O2 or dioxygen's particular formulation is one of the most common elemental compounds on the planet, constituting around 20.8% of the Earth's atmosphere. Let us follow some steps to draw the Lewis structure of chlorine dioxide: Step 1: Find the total valence electrons in one molecule of chlorine dioxide. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. There are two oxygen molecules in chlorine dioxide so the total is 19. The Lewis structure of #"O"_2# gives a misleading impression.. It shows that all the electrons in oxygen are paired, so oxygen should be diamagnetic.. Yet oxygen is paramagnetic.. The correct explanation comes from Molecular Orbital theory.. The atomic orbitals of the #"O"# atoms overlap to form the σ and π orbitals of the #"O"_2# molecule as shown in the diagram above. O2 Lewis Structure. O2 Lewis structure, oxygen is the diatomic molecule and hence two atoms of the elements combine together to form dioxygen. The oxygen has six electrons in its valance shell. They try to gain or share two electrons to complete their octet and to get stability. By using the formula of Q, we can calculate the total electrons
O2 is a chemical formula of Oxygen gas. To determine the polarity of any molecule it needs to look out the Lewis Structure of a given molecule. In the Dioxygen contains two oxygen they are sharing pair of electron and forming a bond and remaining electrons are placed around it. Polarity is determined with the help of the electronegativity value.
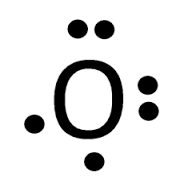
14+ Lewis Dot Structure For O2. We use lewis dot structures based on valence electrons. The lewis dot structure for o2 or dioxygen is as follows: Lewis structure is basically a graphic representation of the electron distribution around an atom. You must draw the lewis dot structure first.
Example 3. Draw the Lewis Dot Structure for Oxygen. Since Oxygen is in Period 2, it can fit a maximum of eight (8) electrons second energy level. Oxygen Group VI, which means it has a total of six (6) valence electrons around the atom. Example A. Determine the total number of valence electrons for C. Carbon is in Group IV, 4 valence electrons
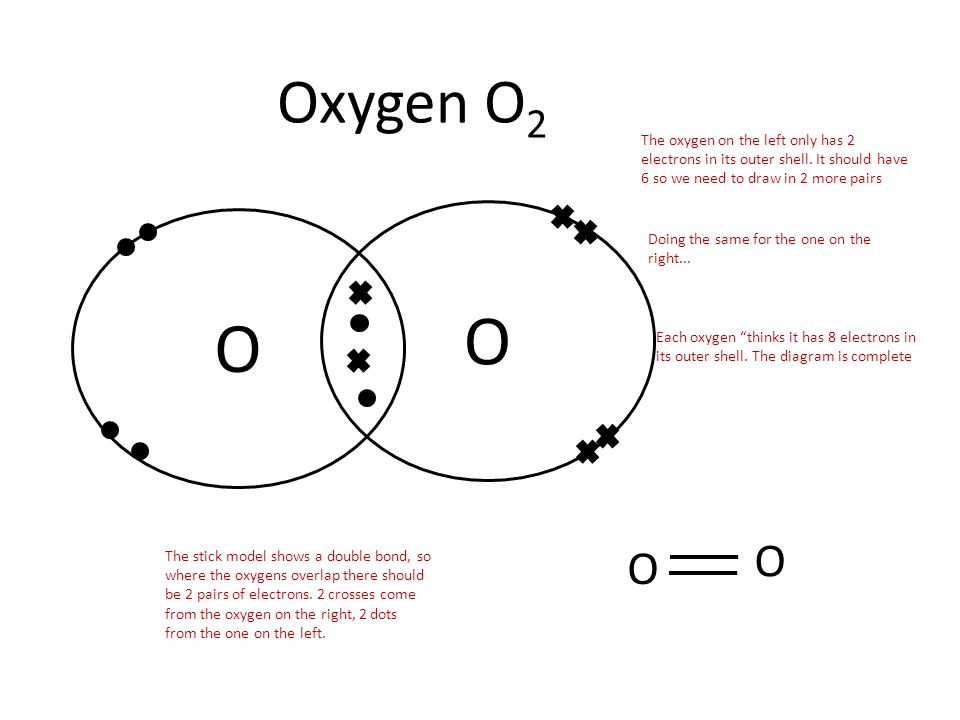
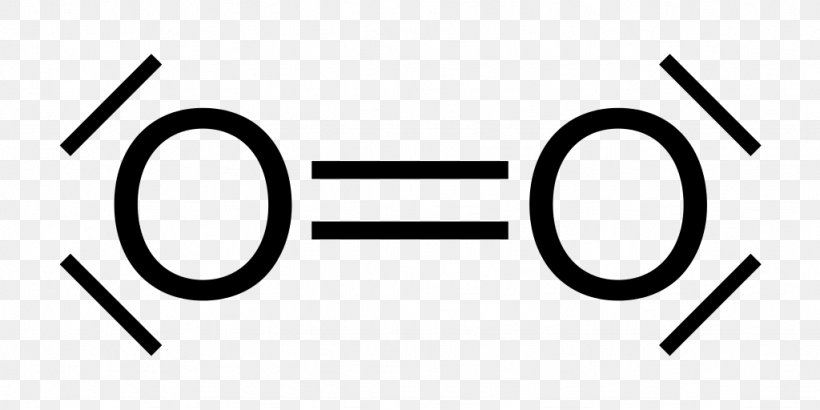
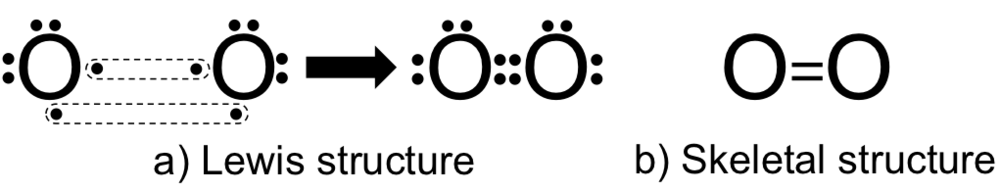
The Lewis structure for oxygen (O2) shows the bond between two oxygen atoms. Each has a total of 6 valence atoms making a sum total of 12. The two oxygen atom can both achieve a stable structure by sharing two pairs of electrons. The double bond they share is denoted by the double lines joining the two atoms.
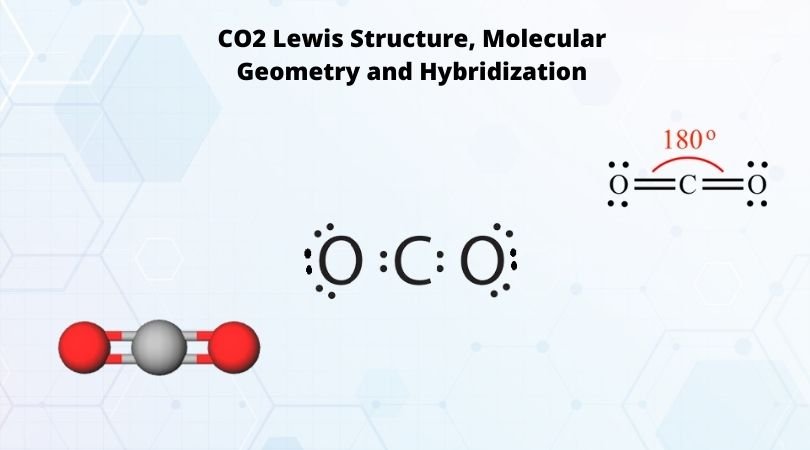
Lewis Structure of Carbon Dioxide ( C O 2). In ( C O 2), oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Step 1- In ( C O 2) we, have C = 4 × 1 = 4 valence electrons.
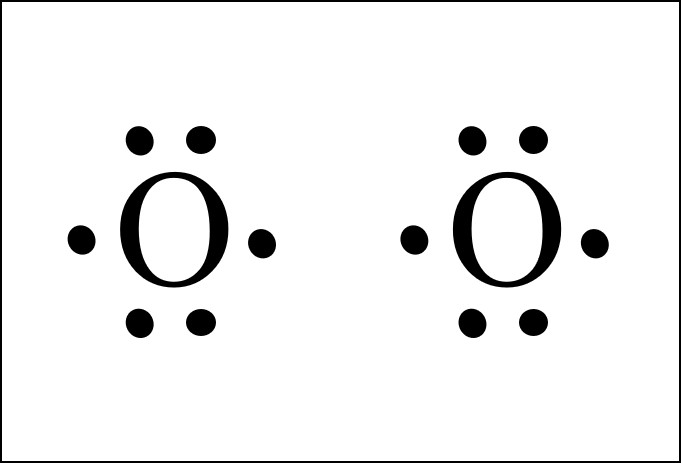
The Lewis structure helps with understanding how electrons are distributed within a compound along with its molecular geometry. Besides this, the lewis structure helps with determining the hybridization of the molecule. Lewis structure of O2. The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
O2 Lewis Structure- Key Points. In the O2 Lewis structure, there is a double bond between two oxygen atoms. O2 is colorless, odorless, and tasteless gas. Density = 1.429 g/L. The boiling point is -183.0 C. Melting point −218.4 °C. Molar mass of O2 is 15.9994 g/mol. Oxygen has two allotropic forms O 2 and O 3.
Answer (1 of 4): The easiest way to them is in steps: Step 1: Count number of total Valance electrons (12 electrons in this case) Step 2: No. of Required electrons (always 8 hence, 16 electrons) Step 3: No. of Bonding Electrons (Required electrons - valence electrons: 4 electrons in this case)...
Draw a diagram: Draw a Lewis diagram of the oxygen molecule in the space below at left. To check your work, turn on. Show Lewis diagram. Draw the correct diagram on the right. Practice diagram: O O. Actual: O O. 4. Practice: Create covalent bonds and stable molecules for the remaining substances. Take a
around each oxygen atom in the dioxygen Lewis diagram above to show the octet rule. The circles do not need to be drawn perfectly circular, but they do need to enclose the correct electrons. See how in this case each oxygen atom shares two of its electrons with the other oxygen atom. So alto-
In nature, the Lewis structure for oxygen exists this way so it could react with other elements and in nature, it is a gas so it exists as O2. As you can see, O2, each oxygen is connected by a.
The Lewis Dot Structure for O2. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for O2, commonly referred to as oxygen gas. Due to oxygen's high electronegativity (affinity for electrons), the pure element is nearly exclusively found in either this state or ozone (O3 - a distinct lewis structure for another post).
Drawing the Lewis Structure for O 2. Viewing Notes: For the Lewis Structure for O 2 you're going to need a double bond in order to for each Oxygen atom to have an octet. That's the only way you can make it work with the twelve valence electrons available.; Be sure that you don't use more than the tweleve valence electrons available.
Draw the ho2 lewis structure polyatomic hydroperoxyl ho2- anion. The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. In order to determine the molecular geometry for H2O observe the Lewis structure of the same.
the Lewis dot diagram is a table used for the elements, and it shows you how many valence electrons there are. What is the Lewis dot structure for Ce? what is the dot diagram for Ce









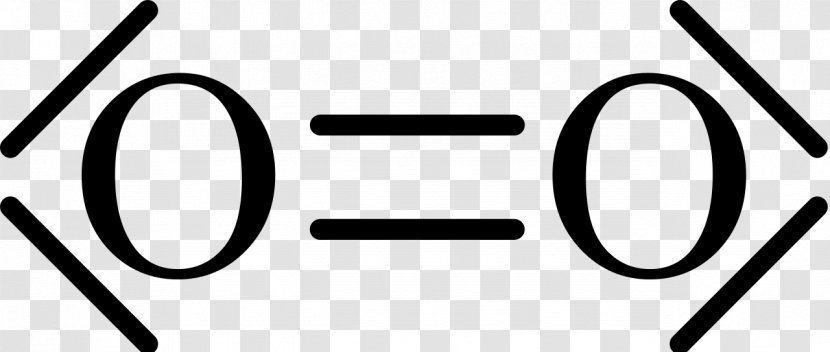













0 Response to "43 Lewis Diagram For O2"
Post a Comment