43 Lewis Dot Diagram For So2
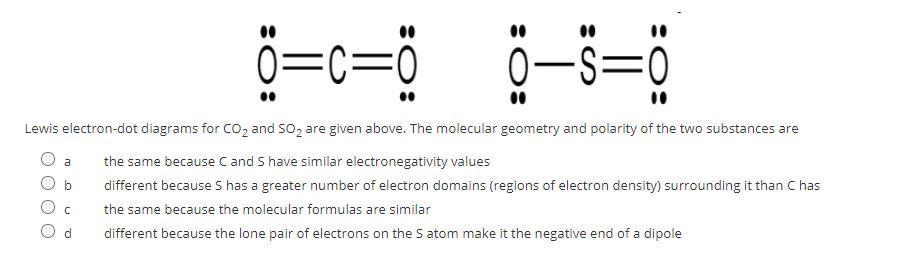
Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial. Answer (1 of 2): Lewis Structure for SO2 (Sulfur Dioxide)||Lewis Dot Structure of SO2 (Sulfur Dioxide) Hello,today I am going to draw the lewis structure for SO2 in just five steps. Step-1: To draw the lewis structure for SO2, we have to find out the valence electrons of sulfur and oxygen firs...
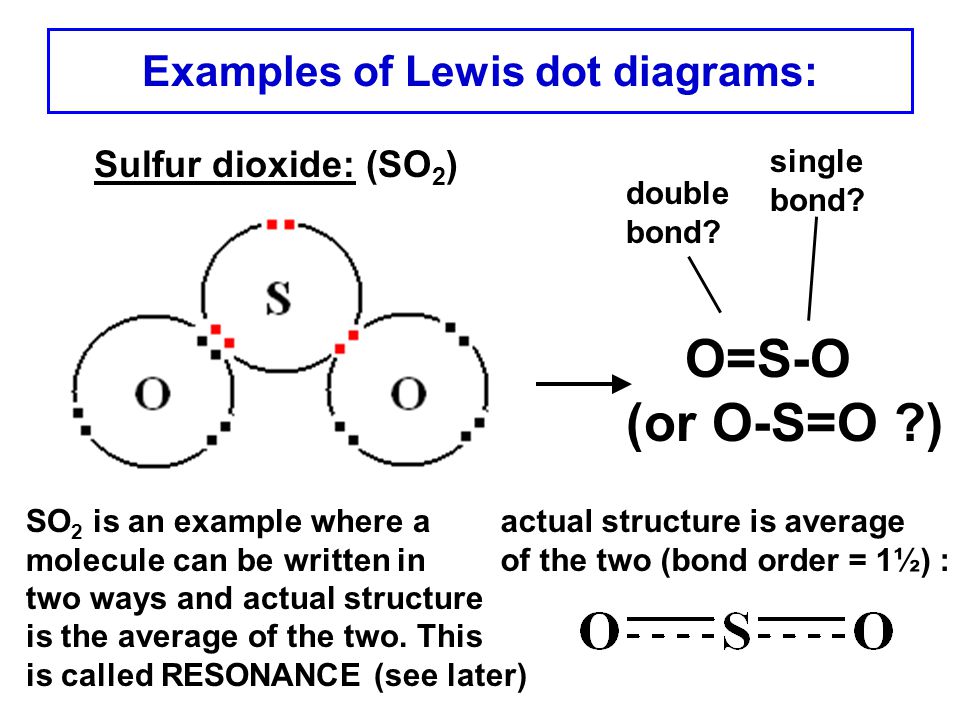
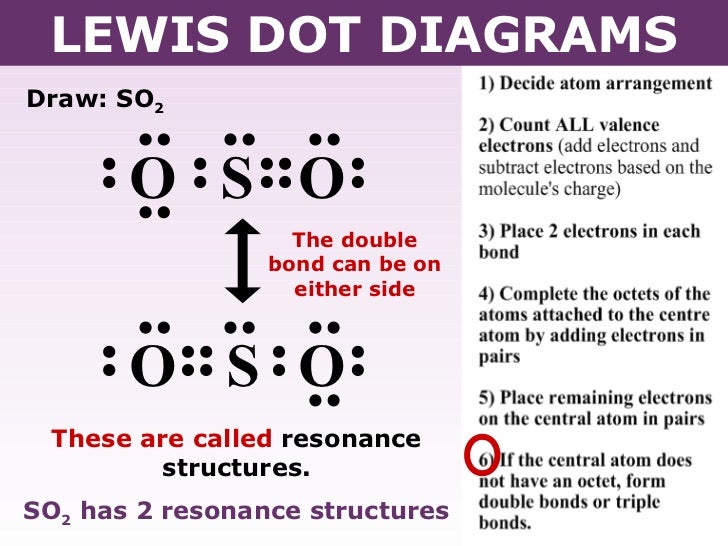
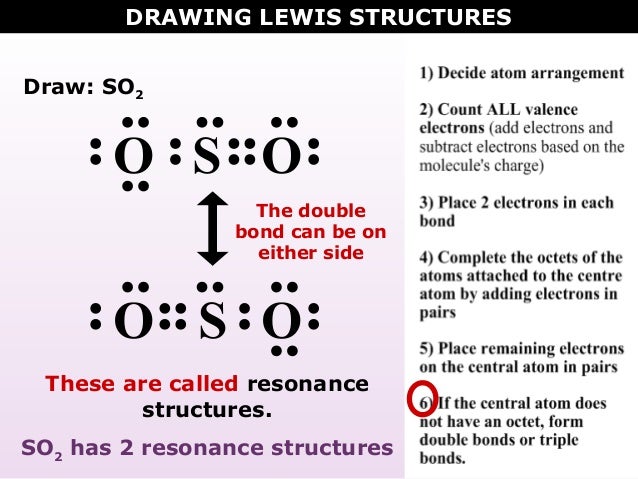
Dot Diagram For So2. Here are the steps I follow when drawing a Lewis structure. Decide which is the central atom in the structure. That will normally be the least electronegative atom (S). * Draw a skeleton structure in which. Well it's hard to draw in a dot cross because of the resonance, but a google from looking at this diagram, i can see.
Lewis dot diagram for so2
CO 2 Lewis Dot structure Is two double bonds Which Going from carbon to the oxygen atoms. In This Every Oxygen Need To bond twice and the carbon atom needs to bond 4 times. Accoridng To Octet Rule The Correct Levis Dot Structure of co2 Is :.. O = C =.. O:. Co2 Lewis Structure. Now We Draw Co2 Lewis Structure . Let us study its lewis structure, geometry, and hybridization. Lewis Structure of Selenium Dioxide (SeO2) Also called the Lewis dot structure, it is a pictorial representation of the behavior and arrangement of valence electrons within an atom. It uses dots to represent valence electrons and lines to show bonds. Here are the steps I follow when drawing a Lewis structure. > 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ("S"). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-S-O". 3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. In this editor, I will.
Lewis dot diagram for so2. Whats the lewis dot structure for carbon dioxide the lewis dot structurerepresenting co2 is refer to the link for an illustration of the lewis dot diagram for carbon dioxide to maxresdefault co2 molecular geometry and lewis structure do lewis structure and molecular geometry confuse you stop worrying and read this simplest explanation regarding. Sulfur dioxide (SO 2) Lewis Structure, Hybridization. Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO 2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO 2, we can determine the hybridization of atoms. Let us study its lewis structure, geometry, and hybridization. Lewis Structure of Selenium Dioxide (SeO2) Also called the Lewis dot structure, it is a pictorial representation of the behavior and arrangement of valence electrons within an atom. It uses dots to represent valence electrons and lines to show bonds. A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth...
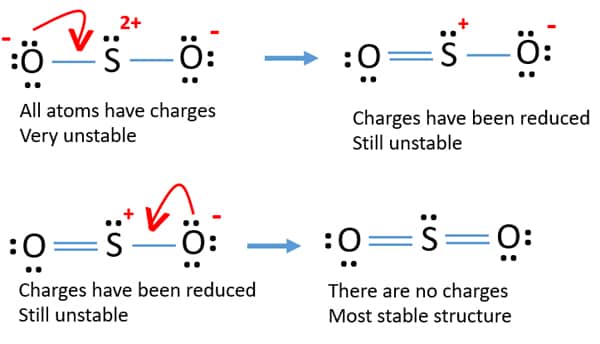
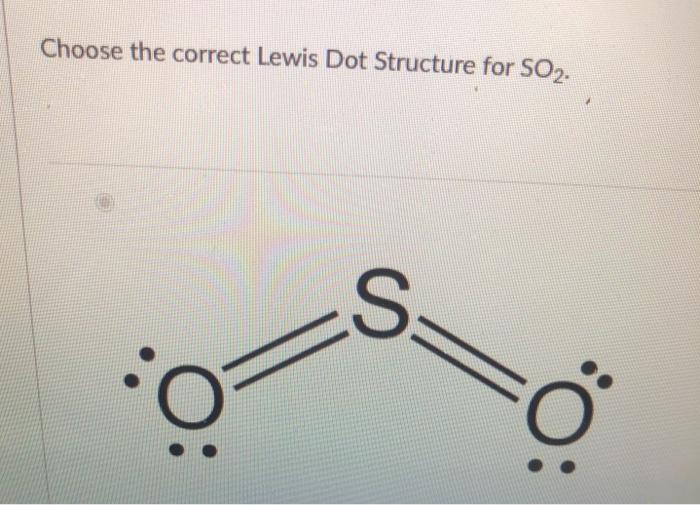
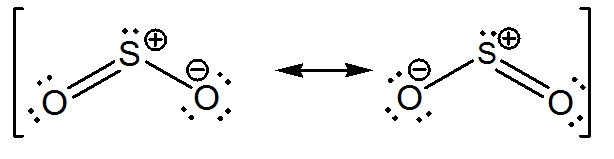
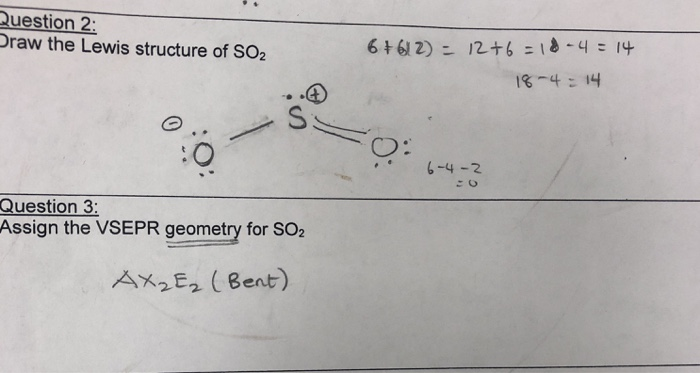
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access. Drawing the Lewis Structure for SO 2. Viewing Notes: The Lewis structure for. What is the electron dot diagram of carbon dioxide? The molecular formula of carbon dioxide is CO2. - The Lewis dot diagram is also called the Lewis electron dot structure, here the valence electrons present in the compound are represented as dots around the atoms. Let's do the SO2 Lewis structure. On the periodic table: Sulfur, 6 valence electrons. Oxygen has 6. We have two Oxygens, though, for a total of 18 valence electrons. We'll put the Sulfur in the middle; it's the least electronegative. Oxygens on the outside, and then we'll use our valence electrons. Here are the steps I follow when drawing a Lewis structure. > 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ("S"). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-S-O". 3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. In this editor, I will.
Electron dot structures of chloryl ClO2+ - An automatic procedure for writing canonical forms, pi an d, lewis dot diagram for chlorine dioxide ion, lewis dot structure for chlorine dioxide ion, resonance structures of clo2+,lewis dot structure of chlorine dioxide cation clo2+, σ bonds in chlorine dioxide cation clo2+, π bonds in chlorine dioxide cation clo2+, calculate the number of. The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur S. It has bent or v shape. To draw the SO2 Lewis structure we have to find out the SO2 valence electrons firstWe express valence electrons as dots in lewis dot structure. CO2 lewis structure contains two oxygen atoms and one carbon atom, connected with the double bond whereas carbon is the central atom, and no lone pair is present on it. But each oxygen in the CO2 lewis dot structure has two lone pairs. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. CO 2 Lewis Dot structure Is two double bonds Which Going from carbon to the oxygen atoms. In This Every Oxygen Need To bond twice and the carbon atom needs to bond 4 times. Accoridng To Octet Rule The Correct Levis Dot Structure of co2 Is :.. O = C =.. O:. Co2 Lewis Structure. Now We Draw Co2 Lewis Structure .
Draw Lewis electron dot structure of SO2. Maharashtra State Board HSC Science (General) 11th. Textbook Solutions 8028. Important Solutions 18. Question Bank Solutions 5552. Concept Notes & Videos 418. Syllabus. Advertisement Remove all ads. Draw Lewis electron dot structure of SO2 - Chemistry.
How to draw the Lewis Structure of SO2 - with explanationCheck me out: http://www.chemistnate
SO2 Lewis structure. To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too. You know that both the Sulphur and Oxygen has six valence electrons each. Click to see full answer.
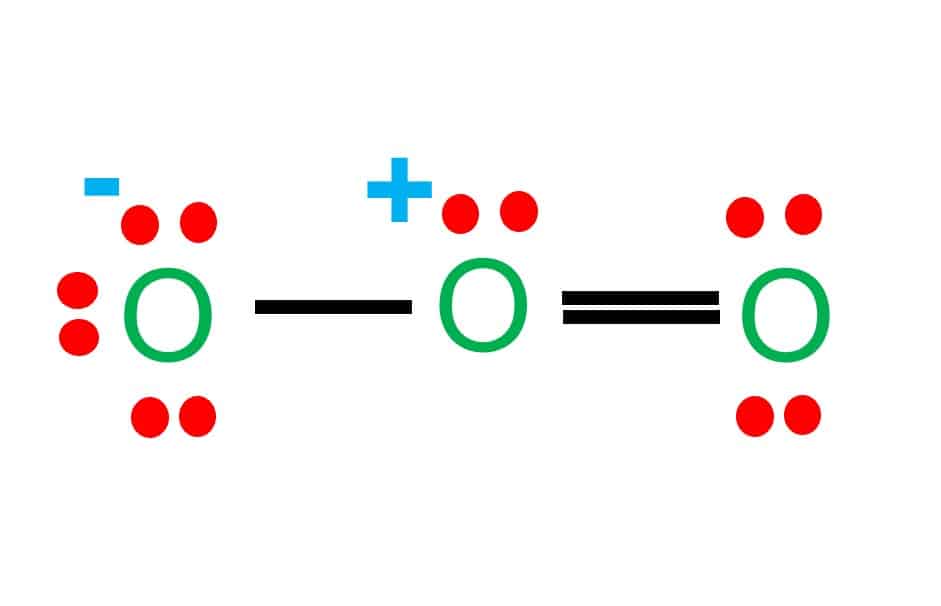
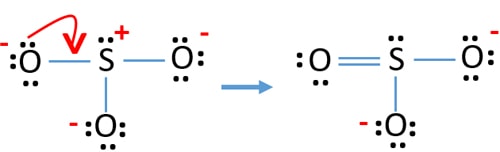
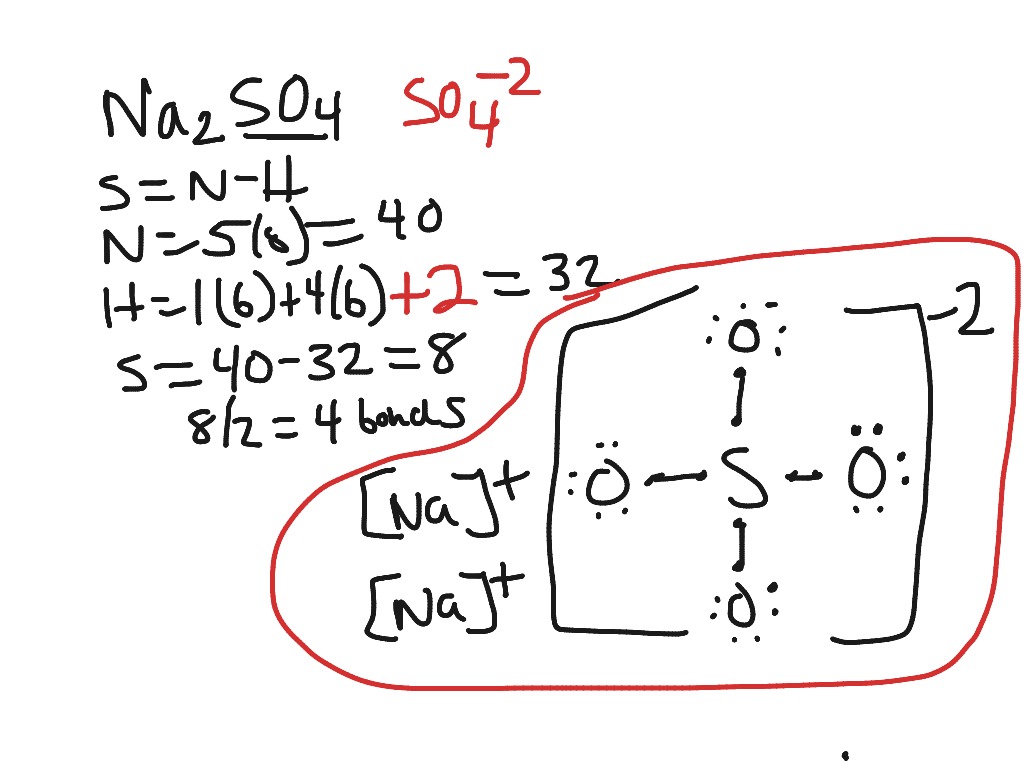
Lewis dot structure of sulfur dioxide. Now let us calculate the formal charge on each atom in the lewis dot structure of SO2 molecule. SO2 formal charge calculations. Now let us check for NO 3 - (nitrate ion) Total valence electrons = 24. Electrons used are as 4 bond pairs and 8 lone pairs =4*2+8*2=24. Hence all 24 valence electrons are used up.
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access to the. Here are the steps I follow when drawing a Lewis structure.
The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make.
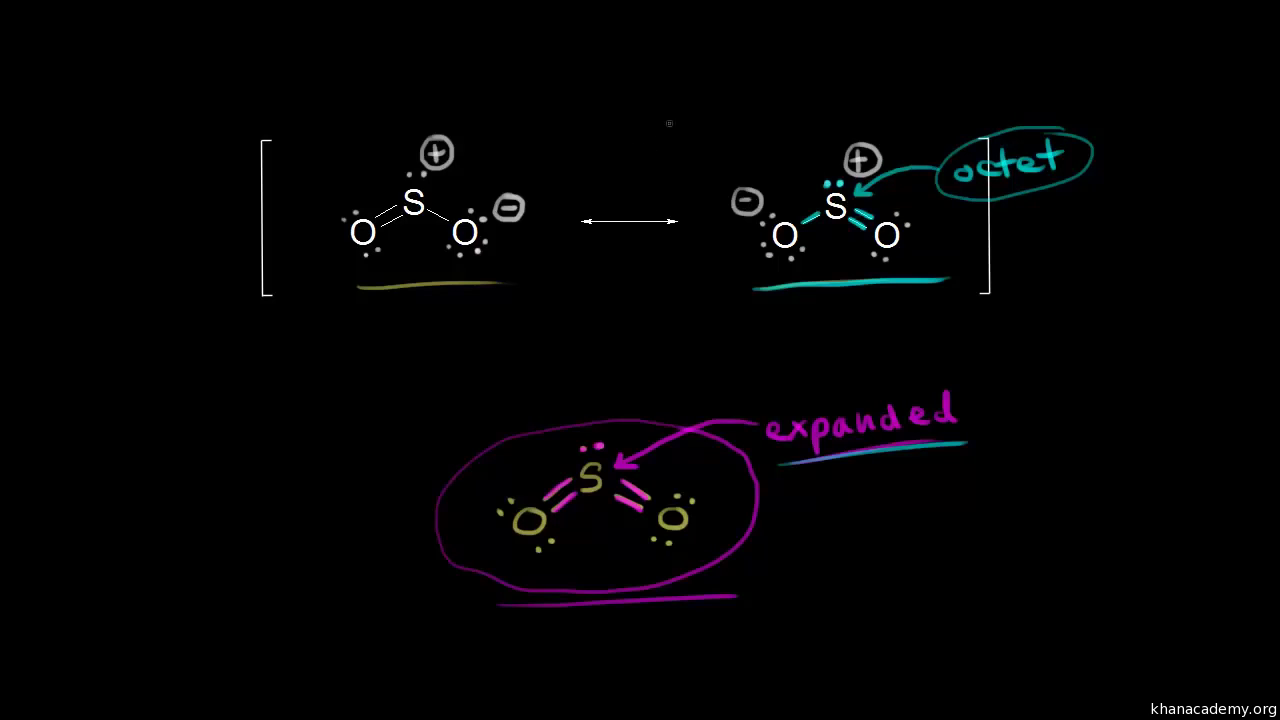
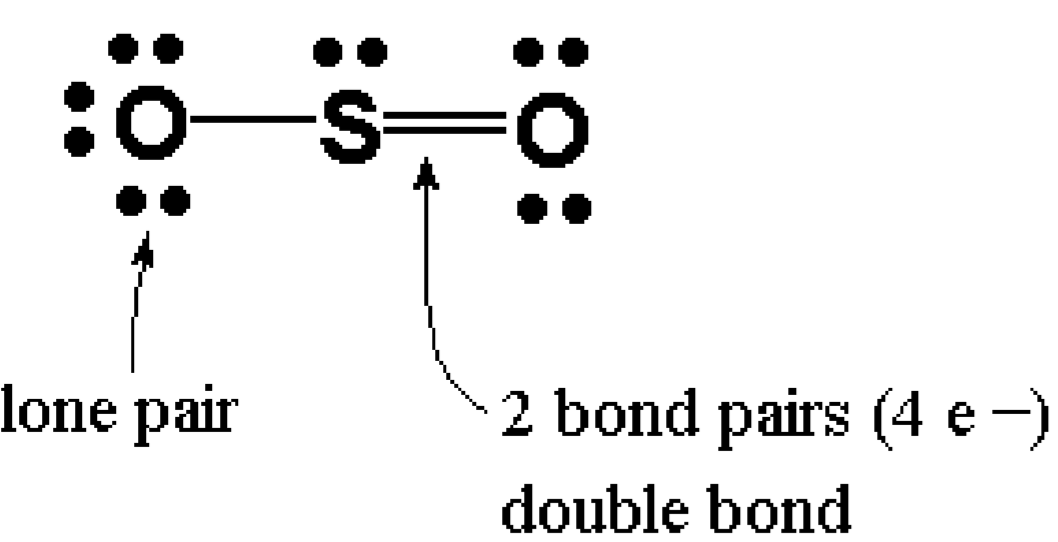
Lewis Dot of Sulfur Dioxide. SO 2. Back. 70 More Lewis Dot Structures. S does not follow the octet rule. It can hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. NOT IN THIS MOLECULE.
By analyzing the Lewis structure of SO2, we can see that the SO2 is asymmetrical because it contains a region with different sharing. The molecular geometry of SO2 has a bent shape which means the top has less electronegativity, and the bottom placed atoms of Oxygen have more of it. So, the conclusion is, SO2 is a Polar molecule.
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
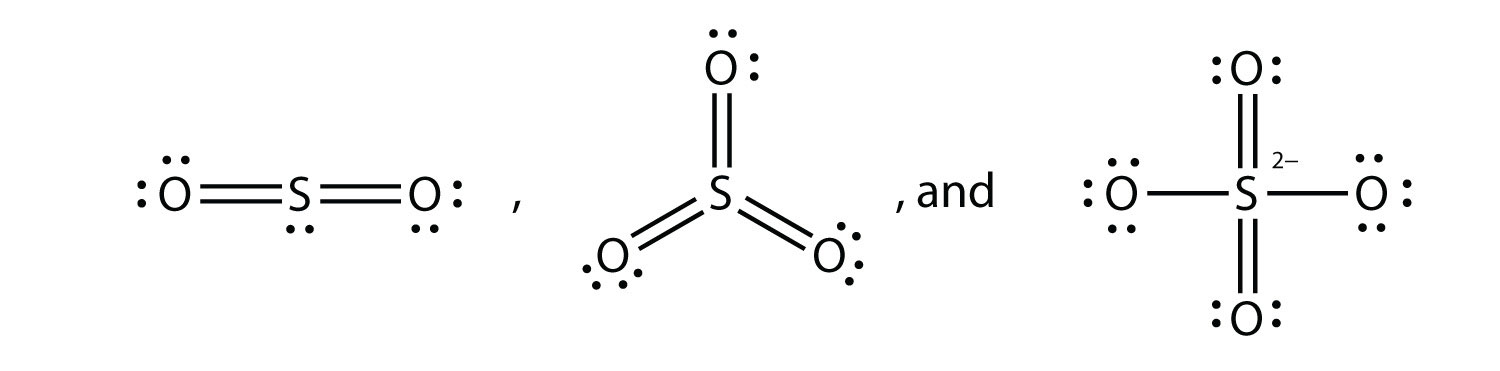
The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
Silicon dioxide (SiO2) lewis structure. As you see in the above SiO2 lewis dot structure, we convert 2 lone pairs of electrons of each oxygen atom to a covalent bond. So, both atoms (silicon and oxygen) have 8 electrons in their valence shell. Hence we got our best and stable Silicon dioxide lewis structure.

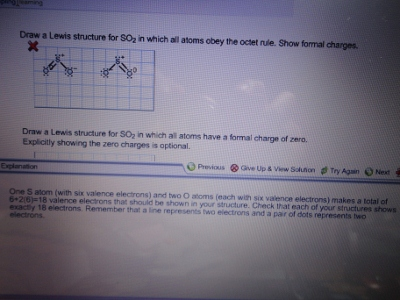
Draw A Lewis Structure For So2 In Which All Atoms Obey The Octet Rule Show Formal Charges Draw A Lewis Structure For So2 In Which All Atoms Have A Formal Charge Of Zero Explicitly Showing The Zero C
Conclusion. SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero.
SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2. The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure. Step-1: Count the valence electrons of atoms.























0 Response to "43 Lewis Dot Diagram For So2"
Post a Comment