39 Diagram Of Salt Dissolved In Water
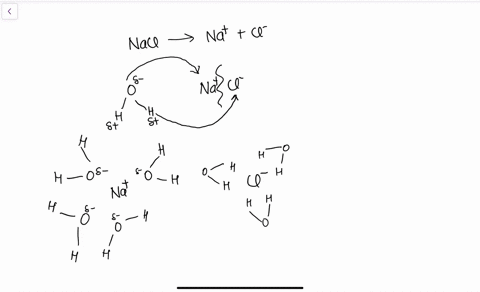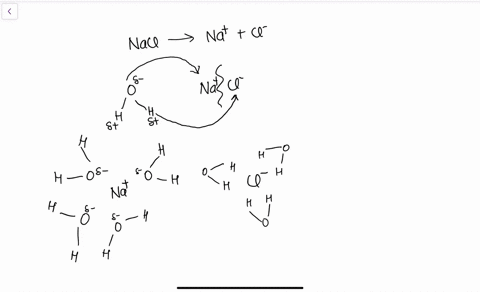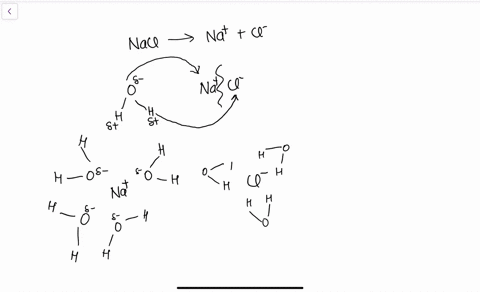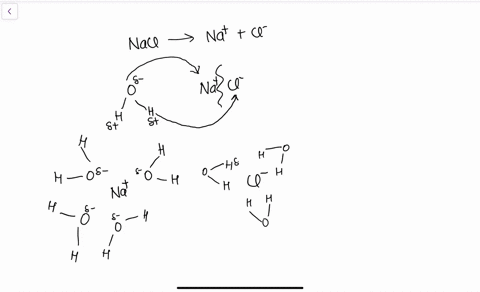
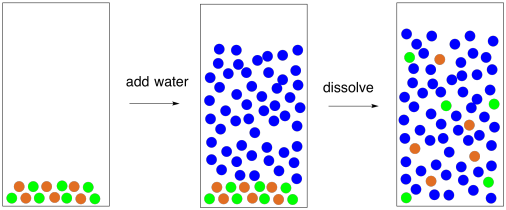
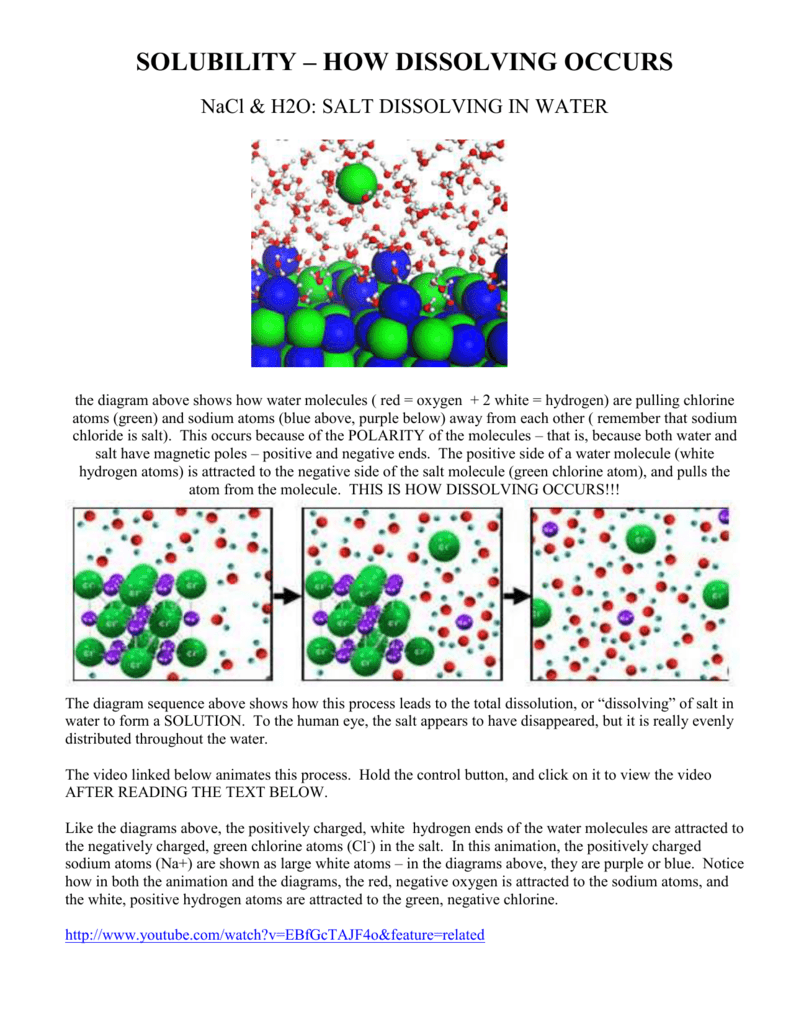
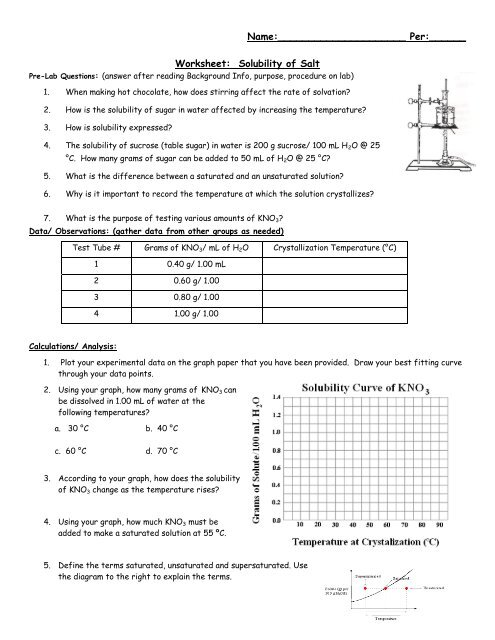
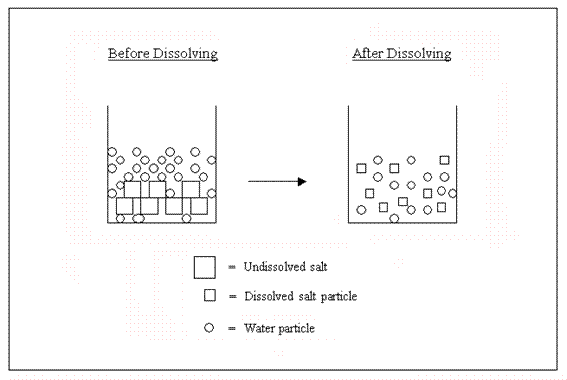
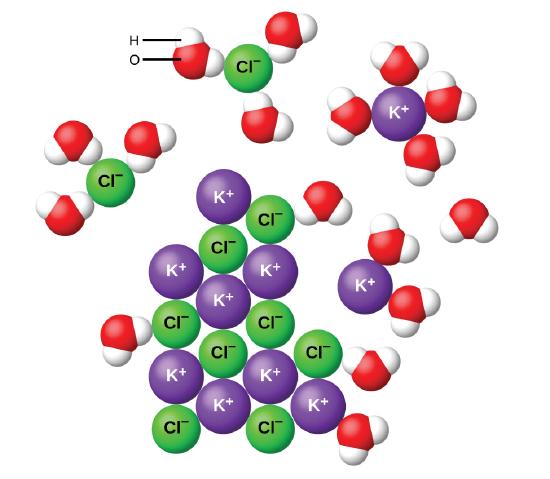
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl –) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed.
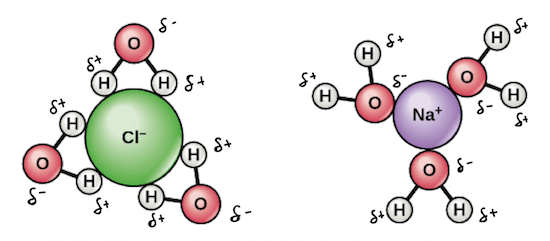
The Cl− ion is larger in size than the Na+ ion. The diagram best represent the hydration is c. because NaCl Ions are separated and H positive Inos is attracted to Cl which are negative. 3.Identify each of the following substances as a strong electrolyte, weak electrolyte, or nonelectrolyte: (a) H2O, (b) KCl, (c) HNO3, (d) HC2H3O2, (e) C12H22O11.

Diagram of salt dissolved in water
For our purposes, we will generally be discussing solutions containing a single solute and water as the solvent. What is a solvent? In crudest terms it is the molecule in the mixture with the highest concentration. That is to say if you had a liter of salt and 2 grams of water. In that case, the salt would be the solvent and the water the solute. Let's take the lines one at a time. The first one to look at is the one in bold green in the next diagram. This line represents the effect of increasing amounts of salt on the freezing point of water. Up to the point where there is 23.3% of salt in the mixture, the more salt the lower the freezing point of the water. Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and chlorine anion). Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does not result in a chemical reaction.
Diagram of salt dissolved in water. If the water in the solution is evaporated, the solute is left behind. The total mass stays the same during dissolving. For example, if 1 g of salt is dissolved in 100 g of water, the mass of salt. Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then dissolving the salt.. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal... You could easily dissolve about 360 g of table salt in a liter of water, but the solubility of calcium carbonate is only about 0.01 grams per liter. That's partly due to the fact that the ions in sodium chloride, Na + and Cl-, have lower charges than the ions in calcium carbonate, Ca 2+ and CO 3 2-.
Salt such as sodium chloride dissolving in water has been considered to be an endothermic reaction. An enthalpy diagram for this process is shown below. Here, in order to break down a lattice and. Figure 11.4. 1: The hydration of (a) a positive ion; (b) a negative ion. When ions are dissolved in water, they attract and hold several water dipoles around them shown in the circular area in the circular area in the center of each part of the diagram. The resolution of this apparent paradox lies in the interactions between ions and the. Answer (1 of 3): Actually, the volume decreases instead of increase (that is the intuitive result) and, makes your water super cold. LOL, right? How it works. NaCl (salt), when dissolved in water, breaks apart into two ions that I won´t write here because since I can´t format properly, they will... Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride).The salt concentration is usually expressed in parts per thousand (permille, ‰) and parts per million (ppm). The United States Geological Survey classifies saline water in three salinity categories. Salt concentration in slightly saline water is around.
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Students will make a 2-D model of a salt crystal and use water molecule cut-outs to show how water dissolves salt. After seeing an animation of water dissolving salt, students will compare how well water and alcohol dissolve salt. They will relate their observations to the structure of salt, water, and alcohol on the molecular level. Objective A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. A salt is insoluble if the concentration of an aqueous solution is less than 0.001 M at room temperature. Slightly soluble salts give solutions that fall between these extremes. Cool water molecules are tighter together and will not allow much salt to dissolve. PROCEDURE: Students will make salt from water. In this lab ordinary table salt will be used, but in reality the "salt" in salt water consists of various other compounds as will be discussed in the post lab. Water can just take so much salt. Have the students.
0.5 g of salt is dissolved in 25 g of water. Calculate the percentage amount of the salt in the solution. Solution: Mass of salt present = 0.5 g Mass of water present in solution = 25 g ∴ Percentage amount of the salt = \(\frac{0.5}{0.5+25} \times 100\) = 1.96%. Question 2. A solution of urea in water contains 16 grams of it in 120 grams of.
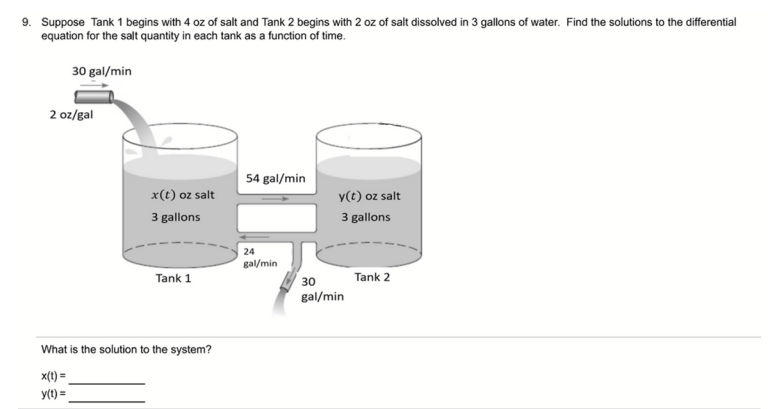
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
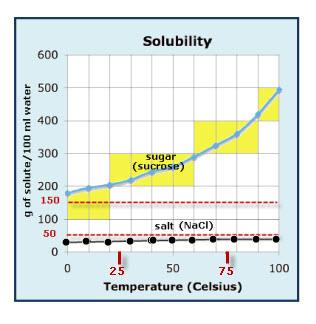
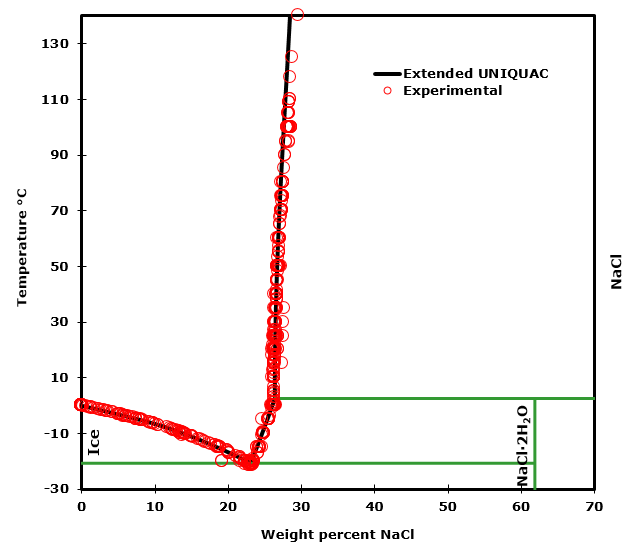
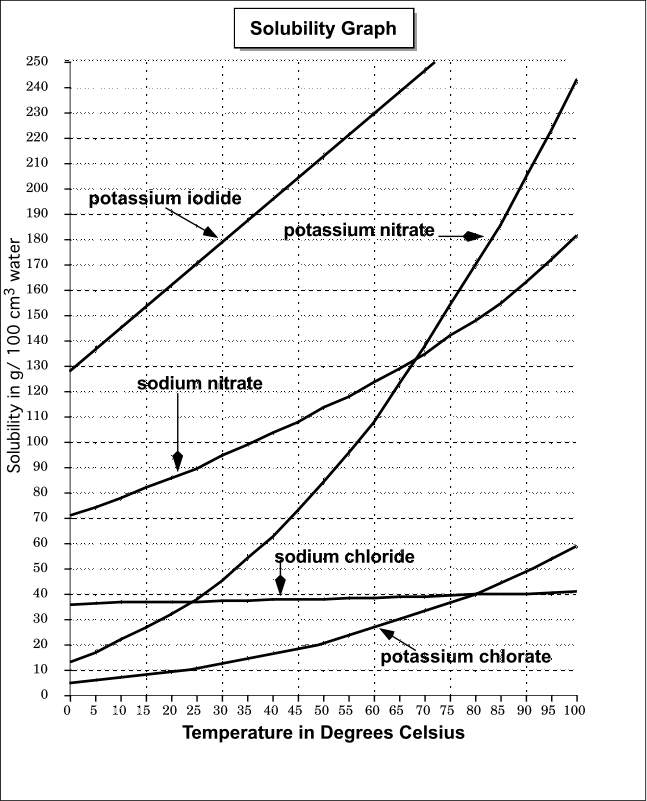
To illustrate the point the solubility diagram for a single salt, such as potassium nitrate, is shown in Fig. 1. In this diagram, the solubility of the salt in water is given by its concentration expressed in molality (mol/kg solvent, where the solvent is water) on the ordinate axis as a function of temperature on the abscissa axis. The black.
Let's take the lines one at a time. The first one to look at is the one in bold green in the next diagram. This line represents the effect of increasing amounts of salt on the freezing point of water. Up to the point where there is 23.3% of salt in the mixture, the more salt the lower the freezing point of the water.
Measure out a fixed amount of water (e.g., 1 liter). Add salt one gram at a time, stirring until it completely dissolves. Repeat until no more will dissolve. With table salt and pure water, you should get around 350-360 grams of salt per liter of water to dissolve. Thanks!
The saturation level is only nominally dependent on the temperature of the water. At 20 °C one liter of water can dissolve about 357 grams of salt, a concentration of 26.3% w/w. At boiling (100 °C) the amount that can be dissolved in one liter of water increases to about 391 grams, a concentration of 28.1% w/w.
A beaker contains two solutions of salt dissolved in water. The two solutions have different concentrations (measured by molarity, M) and are separated by a membrane that is permeable to both salt and water. Side A has 0.2 M of Salt and Side B has 0.9 M of salt.. The diagram below shows a cell with three different membrane transport proteins...
In more formal terms, we can argue that the ion product (Q sp) for the solution is larger than the solubility product (K sp) for AgCl. Q sp = (Ag +)(Cl-) > K sp. The ion product is literally the product of the concentrations of the ions at any moment in time. When it is equal to the solubility product for the salt, the system is at equilibrium.
For our purposes, we will generally be discussing solutions containing a single solute and water as the solvent. What is a solvent? In crudest terms it is the molecule in the mixture with the highest concentration. That is to say if you had a liter of salt and 2 grams of water. In that case, the salt would be the solvent and the water the solute.
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and chlorine anion). Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does not result in a chemical reaction.
Q. Salt dissolved in water. What method would you use to separate this mixture so that you can get the salt AND the water? answer choices. sorting by hand.. Q. The diagram shows the apparatus for separating soil and water. What are the labelled parts? answer choices . A = distillate, B = filtrate.
Weigh out ~5 grams of ammonium nitrate. Record the weight of the salt. Add the salt to the water and stir to dissolve—use a stirring rod, not the thermometer to stir. Observe the temperature changes over time that occur while the salt dissolves. Record the highest or lowest temperature observed as the final temperature.
When salt is dissolved in water, the water dilutes the salt, but the salt also dilutes the water.. The diagram below shows a cell with three different membrane transport proteins. The Na+/K+ Pump is a primary active transporter and the Na+/Waste Co-transporter is a secondary active transporter. Arrows show the direction of net movement of...
This page looks at the phase diagram for mixtures of salt and water - how the diagram is built up, and how to interpret it. It includes a brief discussion of solubility curves. Important: This page is only really designed to be an introduction to the topic suitable for courses for 16 -18 year olds, such as UK A level chemistry.
The correct option is A Salt- Solute. Water- Solvent. In a solution the substance that gets dissolved is called a solute. Whereas, the substance in which the solute gets dissolved is called a solvent. The solute is present in less proportion and the solvent is present in more proportion. Here, salt is getting dissolved in water.
If you mix two substances and the result is a homogeneous mixture, you are dealing with a solution. In the case of table salt mixed with water, Na and Cl atoms, initially bonded together in the form of a crystal, are dissolved by molecules of water. Water is a solvent. The reasons are electrostatic in nature. The cohesion of atoms and molecules derive from electrostatic links between particles.
Solubility Diagram. Show all questions. 1 / 12. At approximately what temperature does the solubility of sodium chloride, NaCl, match the solubility of potassium dichromate, K 2 Cr 2 O 7? 83 ºC. 60 ºC. 50 ºC. 30 ºC. Which salt is LEAST soluble at 0 ºC?
2 Write the balanced reaction for the dissolution of each salt in water, including physical states. The convention is to leave water out of the equation, and simply write the reactant as the solid ionic compound and the product as ions in aqueous solution. Below is an example of a reaction for the dissolution of an ionic compound in water.
















0 Response to "39 Diagram Of Salt Dissolved In Water"
Post a Comment