38 Nickel Titanium Phase Diagram
The nickel base alloys contain gamma phase as a matrix. Pure nickel does not attain an abnormally large elastic modulus or small diffusivity, but the gamma phase is easily reinforced for the extremely severe temperatures and time limits. Few alloys can be employed at 0.85 Tm and for periods about 100,000 hours at lower temperatures. 1.3 Effect of alloying elements. Nickel has a significant effect on the physical and mechanical properties of Cu-Ni alloys (see 2.). While tensile strength, 0.2% proof strength, hot strength, solidus and liquidus temperature and corrosion resistance increase with nickel content, thermal and electrical conductivity decrease.
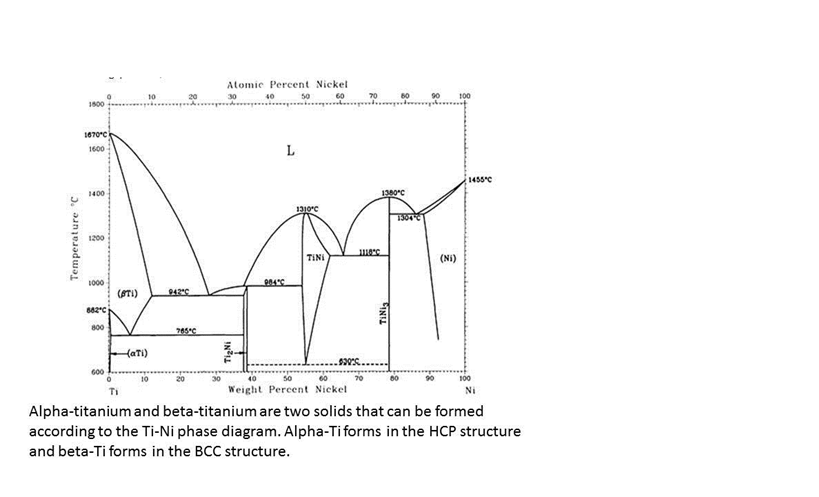
The nickel-titanium phase diagram is shown in Fig. 1. The NiTi equiatomic compound undergoes a martensitic transformation. The martensite phase is soft, has a monoclinic structure, and exhibits the shape memory effect. In addition, the heat of reac-tion for the formation of NiTi is high enough for the CS reaction to take place.
Nickel titanium phase diagram
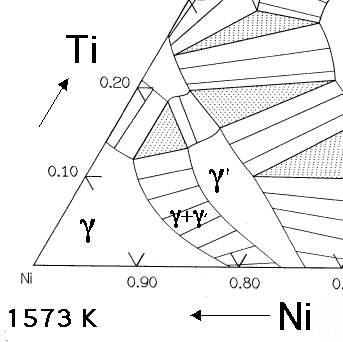
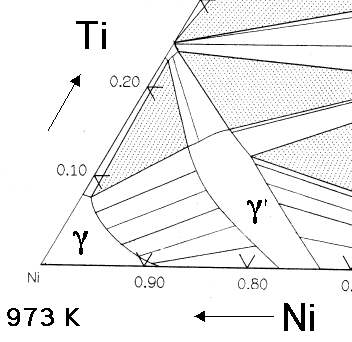
1. Introduction. Titanium alloy micro/meso-scale components have been widely used in the fields of aerospace, weaponry and biomedical engineering due to the advantages of high strength, lightweight, good corrosion resistance, excellent performance under high and low temperature [1,2].However, the micro-forming of titanium alloys is a significant challenge in micro-manufacturing field owing to. phase, 50 or 60 Hz 380 to 480 VAC, 3 phase, 50 or 60 Hz 380 to 480 VAC, 3 phase, 50 or 60 Hz 420-480 VAC, 3 phase, 60 Hz, 112 FLA 400VAC 50Hz Cooling strategy Air Cooled Air Cooled Liquid cooled 8.1 kW Liquid cooled 16.1 kW Liquid cooled 23.7 kW Air or Liquid Air Cooled Operating Temperature 5 to 40 5 to 40 5 to 60 5 to 60 5 to 60 -23 to 46 5. Ternary Diagrams The ternary phase diagrams relevant to the nickel-rich region of the nickel aluminum-chromium-titanium system are shown in figures 1 to 3 and were taken from references 7, 8, and 9. Note that fortunately the investigators did determine tie lines in these systems. As discussed in reference 3, the gamma-gamma prime solvus curve in
Nickel titanium phase diagram. The Al-Ni-Ti system was evaluated by [82Nas]. This evaluation includes additional information from [78Ans], [83Sri], [83Zak], [85Omal], [85Oma2], [85Nas], and [86Edm]. The evaluation by [82Nas] is confirmed for the most part by the recent literature. Download to read the full article text. STRUCTURE OF NICKEL-TITANIUM The crystal structure of NiTi alloy at high temperature ranges (100°C) is a stable, body-centred cubic lattice which is referred to as the austenite phase or parent phase. When it is cooled through a critical transformation temperature range (TTR), the alloy shows dramatic changes in its modulus of elasticity. The γ-phase is a solid solution with a cubic-F lattice and a random distribution of the different species of atoms. Cubic-F is short for face-centred cubic. By contrast, γ' has a cubic-P (primitive cubic) lattice in which the nickel atoms are at the face-centres and the aluminium or titanium atoms at the cube corners. grown in complexity. The Ni-Ti equilibrium phase diagram, TTT diagram, and knowledge about kinetic processes (e.g., diffusion, precipitation, phase transformations) are all invoked for the analysis. Students enjoy the ability to plan parts of the experiment and witnessing the connection between theory and experiment. Bibliography
Titanium solubility in the δ-phase increases gradually as nickel is replaced by ruthenium. The upper titanium border of the δ-phase in the ternary Ti-Ni-Ru system connects the points of the 50Ti-50Ni alloy and the 45Ru-55Ti alloy of the Ti-Ru binary system, corresponding to the maximum titanium solubility in Ti-Ru at subsolidus temperature. The binary phase diagram shown for the copper-nickel alloy indicates that these materials can form both liquid and solid solutions over the full range of composition from Cu to Ni. Above 1728 K, the melting point of pure Ni the alloys ar in the liquid phase. Between 1728 K and 1357 K (the melting point of Cu) the alloys can be either solid or. Nickel titanium, also known as Nitinol, is a metal alloy of nickel and titanium, where the two elements are present in roughly equal atomic percentages.Different alloys are named according to the weight percentage of nickel; e.g., Nitinol 55 and Nitinol 60.It exhibits the shape memory effect and superelasticity at different temperatures.. Nitinol alloys exhibit two closely related and unique. Aug 10, 2020 · The Gibbs free energy diagram of the CO 2 RR on NiPc from DFT calculations showed that the adsorption of CO 2 on the active nickel centre was accompanied by.
' phase has solubility for a number of elements. Earlier work '1) indicated that cobalt would inhabit nickel sites in the y' (Ni3Al) phase, while Cr and MO would tend to occupy both nickel and aluminum sites. Titanium and vanadium tend to occupy aluminum sites. Using this as a basis, a formula: phase, 50 or 60 Hz 380 to 480 VAC, 3 phase, 50 or 60 Hz 380 to 480 VAC, 3 phase, 50 or 60 Hz 420-480 VAC, 3 phase, 60 Hz, 112 FLA 400VAC 50Hz Cooling strategy Air Cooled Air Cooled Liquid cooled 8.1 kW Liquid cooled 16.1 kW Liquid cooled 23.7 kW Air or Liquid Air Cooled Operating Temperature 5 to 40 5 to 40 5 to 60 5 to 60 5 to 60 -23 to 46 5. Oct 24, 2017 · JCE article titled Nickel-Titanium Memory Metal: A “Smart” Material Exhibiting a Solid-State Phase Change and Superelasticity by Gisser et. al. An electronic copy of the JCE paper is posted in the Laboratory menu on yourBlackboard site. lab This assignment is due at the beginning of lab. You will not be allowed to start the Titanium & its Alloys Pure Titanium Pure titanium melts at 1670 C and has a density of 4.51 gcm−3. It should therefore be ideal for use in components which operate at el-evated temperatures, especially where large strength to weight ratios are required. Titanium can catch fire and cause severe damage in cir-
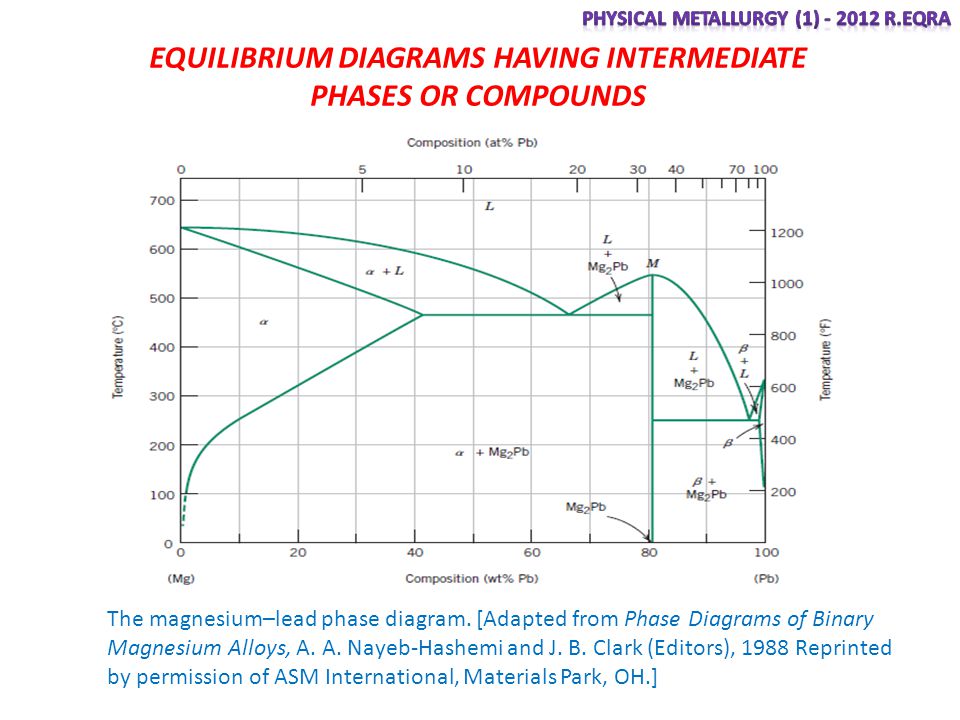
system, the phase diagram usually has the general appearance of that shown in Fig. 3. The diagram consists of two single-phase fields separated by a two-phase field. The boundary between the liquid field and the two-phase field in Fig. 3 is called the liquidus; that between the two-phase field and solid field is the solidus.
Synthesis Descriptions. 10.1007/s11661-011-0623-1. The material used was annealed Ti-55.91wtpct Ni foil in the form of 50500.25mm. It was cut into pieces of 10100.25mm, and the surfaces were ground clean using 600 grit SiC papers.
60NiTi alloy is an intermetallic nickel-titanium alloy with 60 wt.% nickel and 40 wt.% titanium. 60NiTi alloy was manufactured by Xi’an Saite Company and the physical properties of 60NiTi alloy were shown in Table 26.1 [17]. 60NiTi alloy was produced with the forging and hot rolling techniques by consumable electrode melting furnace to ensure.
@article{osti_4832078, title = {THE PHASE DIAGRAM OF THE TANTALUM-NICKEL SYSTEM}, author = {Kornilov, I I and Pylaeva, E N}, abstractNote = {Thermal, microstructural, and x-ray analyses were used to determine the complete phase diagram for the Ta-Ni binary system. The new compounds Ta/sub 2/ Ni, TaNi and TaNi/sub 2/ were formed in Ta-rich alloys by a peritectic reaction at temperatures of 1785.
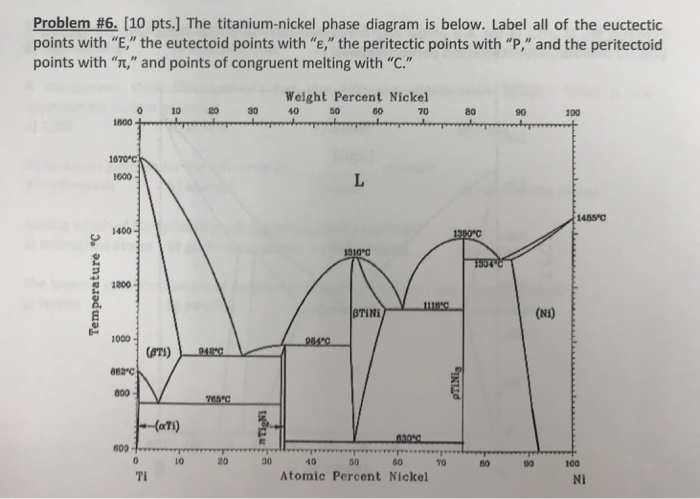
Figure 9.37 is a portion of the titanium-copper phase diagram for which only single-phase regions are labeled. Specify all temperature-composition points at which eutectics, eutectoids, peritectics, and congruent phase transformations occur. Also, for each, write the reaction upon cooling. Question: Figure 9.37 is a portion of the titanium.
Phase diagram. This type of phase transformation is known as spinodal decomposition, and can be illustrated on a phase diagram exhibiting a miscibility gap. Thus, phase separation occurs whenever a material transitions into the unstable region of the phase diagram.
Alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties.Alloy steels are broken down into two groups: low alloy steels and high alloy steels. The difference between the two is disputed. Smith and Hashemi define the difference at 4.0%, while Degarmo, et al., define it at 8.0%.
TITANIUM-NICKEL PHASE DIAGRAM. Technical Report Nielsen, J P ; Margolin, H. STUDIES AND EXPERIMENTAL INVESTIGATIONS FOR THE DEVELOPMENT OF PHASE DIAGRAMS OF THE TITANIUM-CHROMIUM AND TITANIUM-COPPER ALLOY SYSTEMS: PART 2. THE TITANIUM-COPPER AND THE TITANIUM-CHROMIUM PHASE DIAGRAMS. Technical Report Joukainen, A S ; Cuff, F B.
C) is a metastable phase and after long term treatment decompose to form a-ferrite and carbon: Fe3C 3Fe(S) + C(graphite) • Slow cooling and addition of some elements (e.g. Si) promote graphite formation • Properties of cast irons are defined by the amount and microstructure of existing carbon phase. •Equilibrium iron-carbon phase diagram
The Cu-Ni-Ti system was reviewed by [1990Gup] with literature available until 1988. Subsequently, several new results were published and some Russian literature not available earlier could be obtained. This update on the Cu-Ni-Ti system is based on the available published literature up to 1997.
Titanium Alloys. Titanium alloys in turn are divided into α, α+β, and β alloys, depending on the crystal lattice at room temperature, where α phase consists of a hexagonal close-packed structure, and β of a body-centered cubic packing. From: Encyclopedia of Interfacial Chemistry, 2018. Related terms: Aluminum Alloys; Corrosion; Corrosion.
Ternary Diagrams The ternary phase diagrams relevant to the nickel-rich region of the nickel aluminum-chromium-titanium system are shown in figures 1 to 3 and were taken from references 7, 8, and 9. Note that fortunately the investigators did determine tie lines in these systems. As discussed in reference 3, the gamma-gamma prime solvus curve in
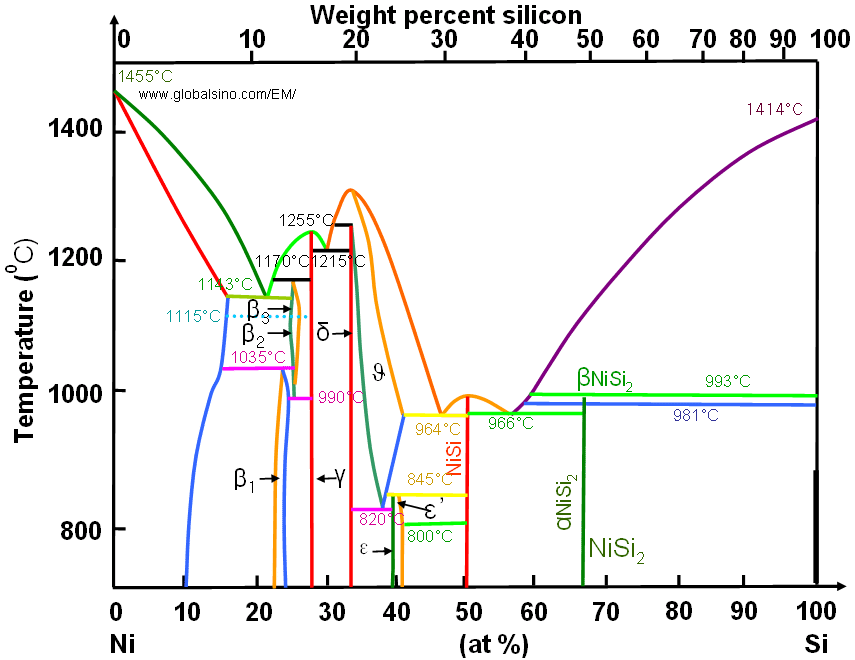
These systems have different behaviours. In fact, while titanium can form the silicide, the carbide and the ternary phase, nickel can only form the silicide. The ternary phase diagrams of the two different systems are reported in Fig. 1,. It can be observed that in the case of Ni reaction on SiC, the only stable silicide phase is the Ni 2 Si.
phase diagram (8). From-the phase diagram it can be seen that many intermetallic compounds including Ni3Ti and NiTi can form: in a nickel/a matrix titanium diffusion couple, which is consistent with our observations. The observed structural stability of the nickel/ 8-titanium diffusion couple is also consistent with phase equilibria
Titanium and zirconium are metallurgically similar. The latter also forms hydrides. Zr-Ti Laves phase Ti 0.24 Zr 0.76 (Ni 0.55 Mn 0.3 V 0.065 Fe 0.085) 2.1 has been found to reversibly accommodate nearly 1.5 wt% of hydrogen, with a battery rating of some 440 mAh g-1. Specific-alloys α-alloys
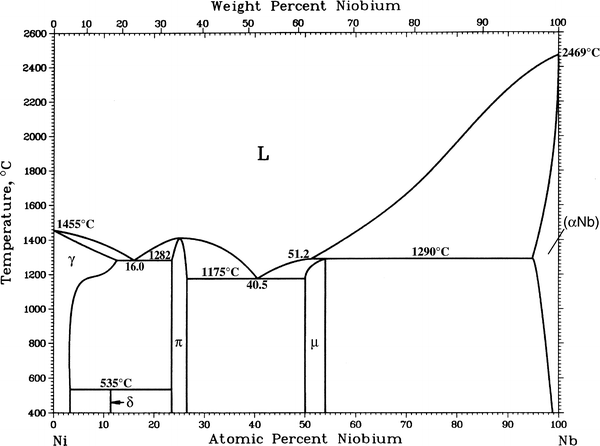
Request PDF | Niobium - Nickel - Titanium | This document is part of Volume 11 `Ternary Alloy Systems: Phase Diagrams, Crystallographic and Thermodynamic Data', Subvolume E `Refractory Metal.
Phase transformations in nickel-rich nickel-titanium alloys : influence of strain-rate, temperature, thermomechanical treatment and nickel composition on the shape memory and superelastic characteristics
The early phase diagrams of the Cu-Ti system showed the terminal FCC Cu-rich solid solution (α) to be in equilibrium with a Cu 3 Ti phase characterized by a space group Pnmm. However, a Au4Zr-type structure was reported and this orthorhombic phase Cu 4 Ti exhibits the space group Pnma.
Download scientific diagram | Titanium-Nickel binary phase diagram from publication: Sintering and reaction behavior in Ni-Ti powder mixtures | Conference code.
A hybrid equilibrium phase diagram for Nb-Ti combining the experimentally determined high temperature phase boundaries of Hansen et al ( ) with the calculated low temperature phase boundaries of Kaufman and Bernstein ( ) modified by Moffat and Kattner ( ). Also shown is the martensite transformation curve (Ms) of Moffat and Larbalestier (). IIIa.
Ti2Ni crystallizes in the cubic Fd-3m space group. The structure is three-dimensional. there are two inequivalent Ti sites. In the first Ti site, Ti is bonded in a 12-coordinate geometry to six equivalent Ni atoms. All Ti-Ni bond lengths are 2.48 Å. In the second Ti site, Ti is bonded in a 2-coordinate geometry to two equivalent Ni atoms.
1. Introduction. Titanium alloy micro/meso-scale components have been widely used in the fields of aerospace, weaponry and biomedical engineering due to the advantages of high strength, lightweight, good corrosion resistance, excellent performance under high and low temperature [1,2].However, the micro-forming of titanium alloys is a significant challenge in micro-manufacturing field owing to.
Y. Ma and A.J. Ardell, The (γ+γ′)/γ′ Phase Boundary in the Ni-Al Phase Diagram from 600 to 1200 °C, Z. Metallkd., Vol 94 (No. 9), 2003, p 972–975 Google Scholar 2005Rag:
Phase diagram of Ni-Ti (nickel-titanium) system. January 2016. DOI: 10.1007/978-3-642-24977-8_70. In book: Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys (pp.125-125)
The Ti-Si-N-O quaternary phase diagram • Entire phase diagram can be calculated by taking into account all possible combinations of reactions and products • 4 ternary diagrams of Ti-Si-N, Ti-N-O, Ti-Si-O and Si-N-O were evaluated • additional quaternary tie lines from TiN to SiO 2 and Si 2N 2O A.S.Bhansali, et al., J.Appl.Phys. 68(3.























0 Response to "38 Nickel Titanium Phase Diagram"
Post a Comment