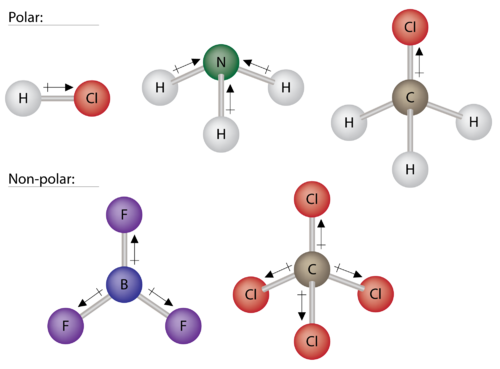
39 Which Diagram Best Represents A Polar Molecule
45.Which diagram best represents a polar molecule? Base your answers to questions 46 through 48 on the information below. In 1864, the Solvay process was developed to make soda ash. One step in the process is represented by the balanced equation below. Which diagram best represents a polar molecule? answer choices. alternatives . answer explanation . Tags: Topics: Question 7 . SURVEY . Ungraded . 60 seconds . Report an issue . Q. Which structural formula represents a nonpolar symmetrical molecule? answer choices . alternatives . answer explanation.
Which electron dot formula represents a polar molecule? The geometric shape of a CH4 molecule distributes the charges symmetrically. Which statement explains why a molecule of CH4. Which diagram best illustrates the hydration of sodium ions in an aqueous solution? [The
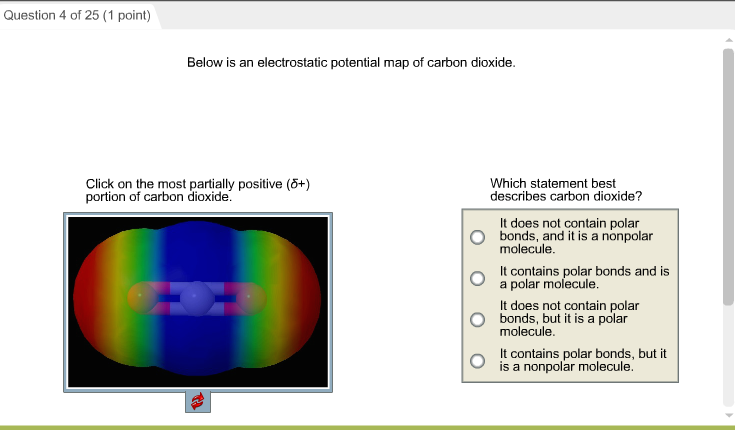
Which diagram best represents a polar molecule
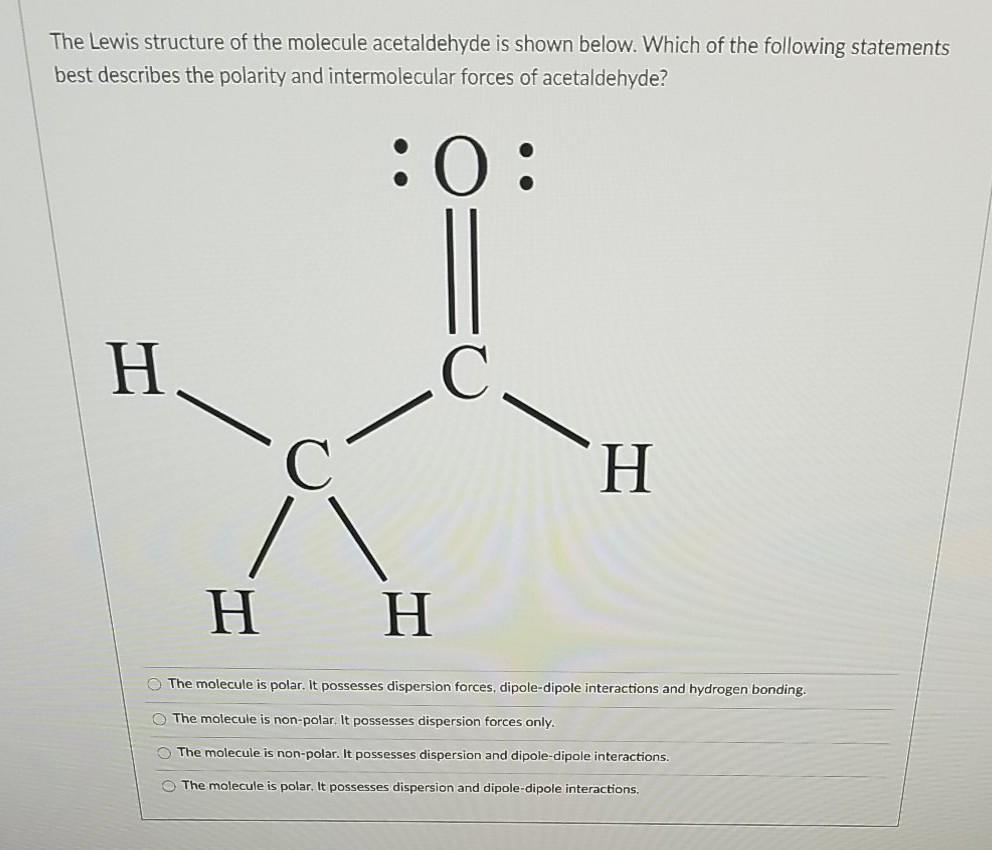
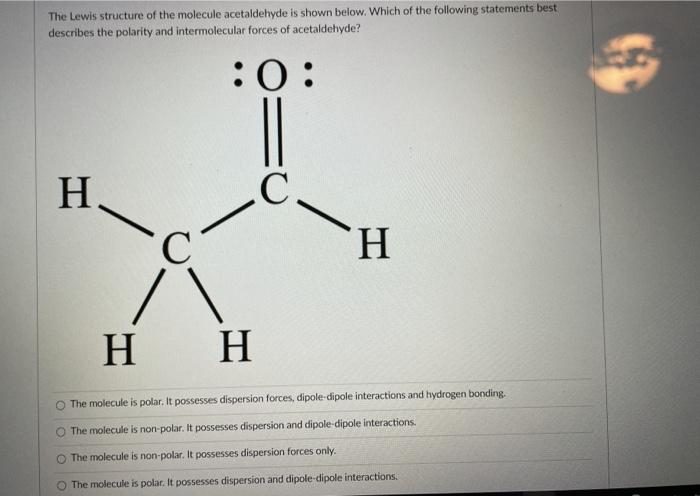
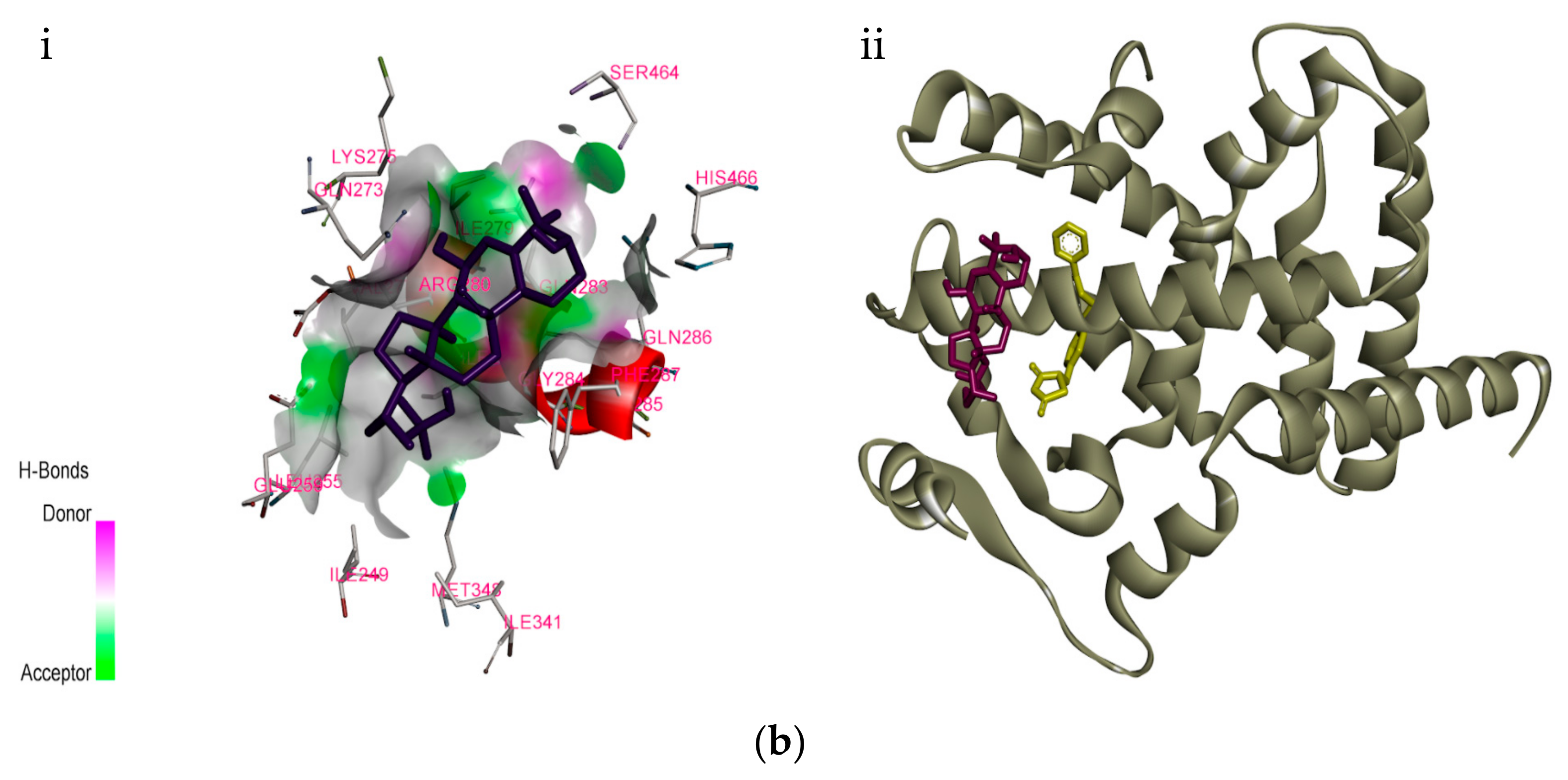
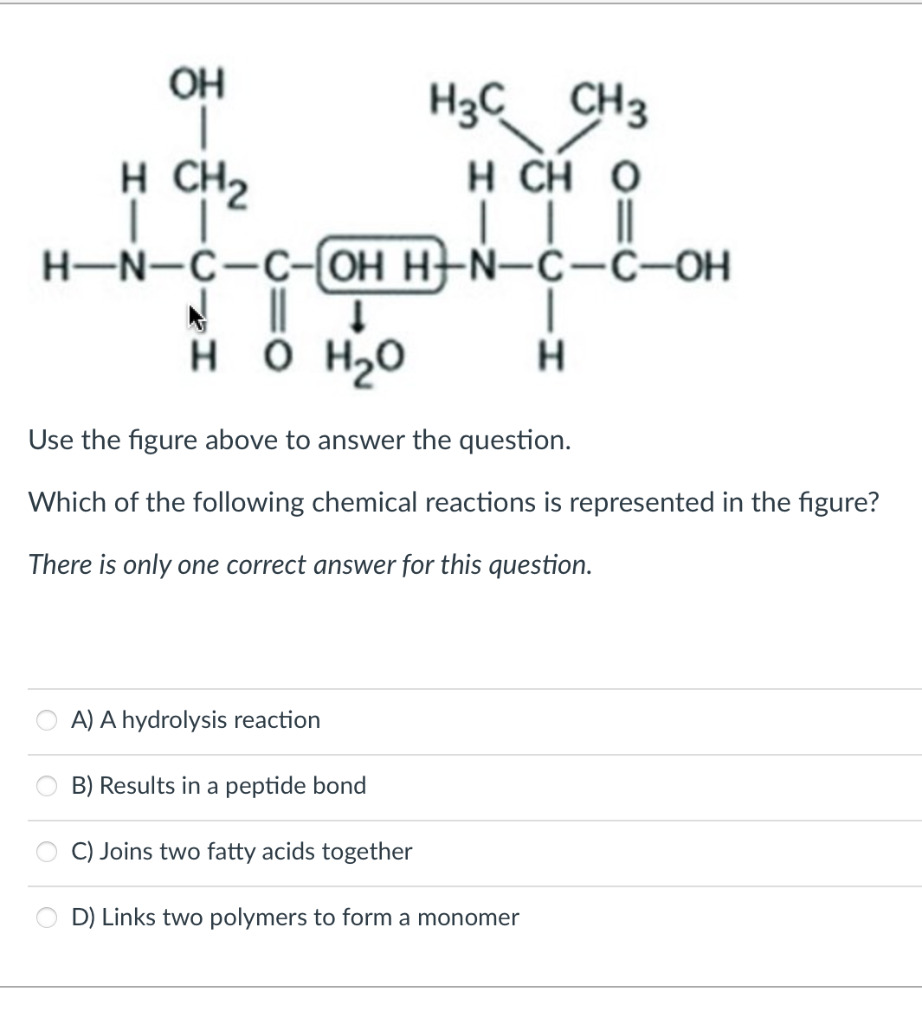
This diagram of CH 4 illustrates the standard convention of displaying a three-dimensional molecule on a two-dimensional surface. The straight lines are in the plane of the page, the solid wedged line is coming out of the plane toward the reader, and the dashed wedged line is going out of the plane away from the reader. For cations subtract one electron for each unit of positive charge. 1h2o 2ccl4 3nh3 4h2 14which formula represents a nonpolar molecule containing. A i 2 b co 2 c nh 3 d h 2o 16 which diagram best represents a polar covalent molecule. 1h2 2h2o 3co2 4ccl4 12which formula represents a polar molecule. Also, by looking at the lewis diagram of acetic acid, its structure doesn’t seem to appear symmetrical, which means, it has unequal or unsymmetrical sharing of valence electrons. This unequal distribution of charge generates a net dipole moment which makes the CH3COOH molecule polar in nature.
Which diagram best represents a polar molecule. Which electron dot diagram represents a polar molecule. 1 n2o 2 so 2 3 cacl 2 4 hcl 28 what type of bonding is found in the molecule hbr. Water is a polar molecule and it therefore dissolves other polar molecules or ionic compounds. Which diagram best represents a polar molecule. The bond between which two atoms is most polar. 7.Given the formula representing a molecule: Which statement explains why the molecule is nonpolar? A)O2 B)CO2 C)NH3 D)CH4 8.Which formula represents a polar molecule? A)symmetrical and polar B)symmetrical and nonpolar C)asymmetrical and polar D)asymmetrical and nonpolar 9.Which phrase describes the distribution of charge and the polarity of a. 1. Write the Lewis structure of boron trifluoride. Ans: 2. (TRUE/FALSE) The bond in F 2 is described as polar covalent. Ans: False 3. Write a Lewis structure for the chlorate ion, ClO 3 -, that obeys the octet rule, showing all non-zero formal charges, and give the total number of resonance structures for ClO 3 - that obey the octet rule. Ans: 30) The diagram below represents a water molecule. This molecule is best described as A) nonpolar with nonpolar covalent bonds B) polar with nonpolar covalent bonds C) nonpolar with polar covalent bonds D) polar with polar covalent bonds
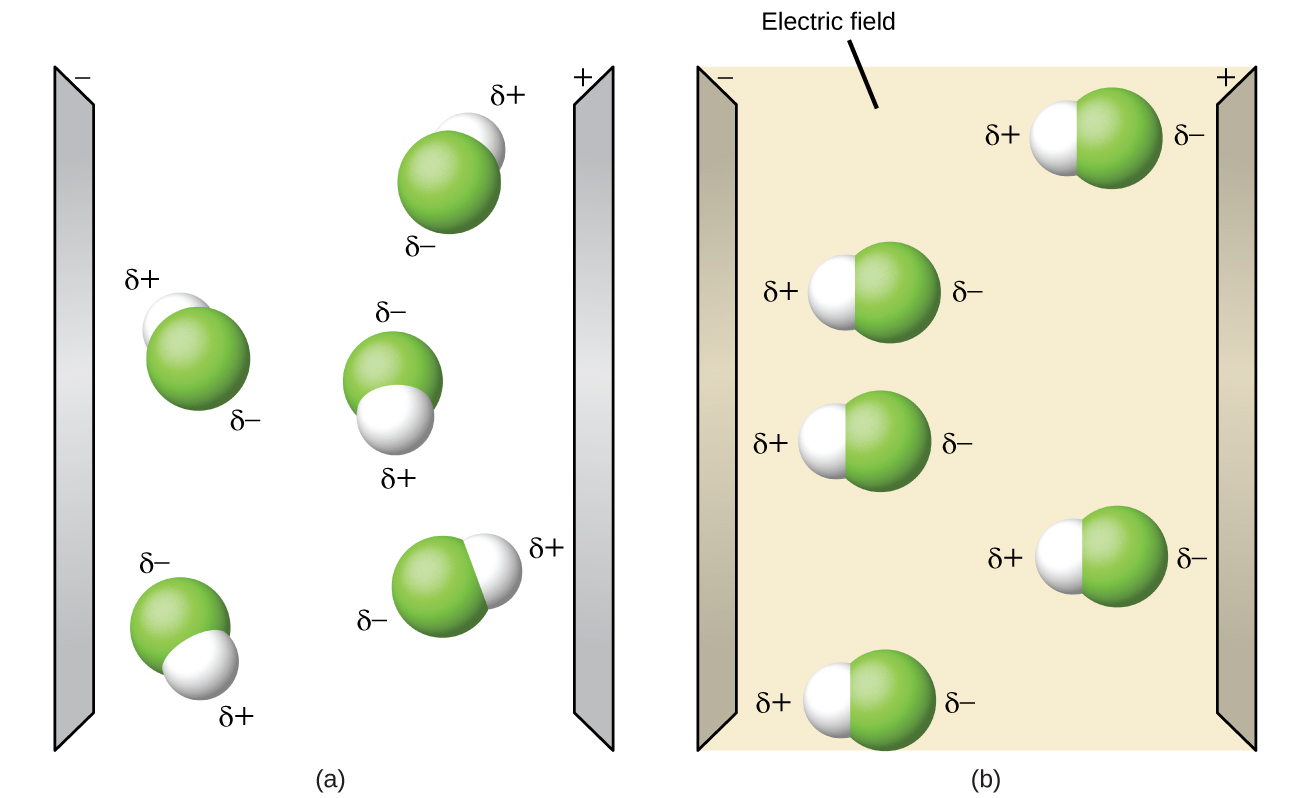
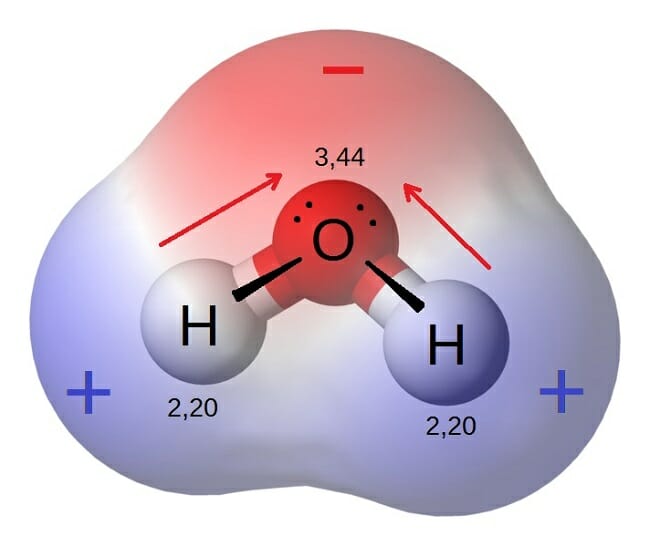
The electrons in a bond between two iodine atoms i2 are shared. The data table below represents the properties determined by the analysis of substances A B C and D. Check also: diagram and which diagram best represents a polar molecule 28A i 2 b co 2 c nh 3 d h 2o 16 which diagram best represents a polar covalent molecule. 16. diagram best represents a polar molecule? B) ) lower average etic energy i C) weaker intermolecular forces of attraction than iodine D) higher average kinetic energy than iodine C) D) 22. Which molecule is polar and contains polar B) CC14 17. Which of the following compounds has the C) NH3 D) C02 A) H20 w represents B) H2Te C) H2S D) H2Se At the time of publication, it represented the best available science. By Steve Graham, Claire Parkinson, and Mous Chahine Design by Robert Simmon October 1, 2010 Viewed from space, one of the most striking features of our home planet is the water, in both liquid and frozen forms, that covers approximately 75% of the Earth’s surface. A polar molecule, on the other hand, is already blessed with electric dipoles and this dipole is not induced. This dipole exists due to the bonds and the structure of a polar molecule. But we cannot utilize this already existing dipole moment right away. Due to thermal agitation, the dipoles in a polar material are oriented randomly.
You are watching: Which structure represents a nonpolar molecule? The better the electronegativity difference, the much more ionic the link is. Bonds the are partially ionic are called polar covalent bonds. Nonpolar covalent bonds, through equal share of the bond electrons, arise once the electronegativities of the 2 atoms room equal. Which of the diagrams above best represents the CH2O molecule, and why? D) Diagram 2, because all atoms have a formal charge of 0. Based on formal charges, which of the following is the best Lewis electron-dot diagram for H3NO? The diagram below represents a hydrogen fluoride molecule? Which diagram best represents a polar molecule? Which is the correct electron dot formula for a chlorine molecule? H2, CO2 and CCl4 are all symmetrical, thus are non-polar. H2O is structurally bent. It is polar. Which electron dot diagram represents a polar molecule Brainly? Interlocking circles are seen in the electron dot diagram that represents a molecule that has a polar covalent bond. Which formulas represent two polar molecules? Answer Expert Verified.
which of the following Lewis diagrams best represents the bonding in the N2O molecule, considering formal charge? ••N---N-O•••••• the BF3 molecule is nonpolar, whereas the NF3 molecule is polar. what accounts for the difference in polarity?
Which electron dot diagram represents h2. 1polar covalent 2nonpolar covalent 3ionic 4electrovalent 10which type of bond is present in a water molecule. Count the total number of valence electrons in the molecule or polyatomic ion. 1the shape of the co2 molecule is symmetrical. A i 2 b co 2 c nh 3 d h 2o 16 which diagram best represents a polar.
Interlocking circles are seen in the electron dot diagram that represents a molecule that has a polar covalent bond. musashixjubeio0 and 9 more users found this answer helpful. heart outlined. Thanks 6. star. star. star half outlined. star outlined. star outlined.
45.Which diagram best represents a polar molecule? Base your answers to questions 46 through 48 on the information below. In 1864, the Solvay process was developed to make soda ash. One step in the process is represented by the balanced equation below.
Which diagram best represents a polar molecule? Which is the correct electron dot formula for a chlorine molecule? Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper. This symbol represents the nucleus of the atom and each of the four sides represents an orbital.
Also, by looking at the lewis diagram of acetic acid, its structure doesn’t seem to appear symmetrical, which means, it has unequal or unsymmetrical sharing of valence electrons. This unequal distribution of charge generates a net dipole moment which makes the CH3COOH molecule polar in nature.
To identify an electron dot diagram with a polar molecule look for highly electronegative atoms to be present like F, O, Cl, N, Br, and I. Wiki User ∙ 2014-06-04 23:59:23
The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond.
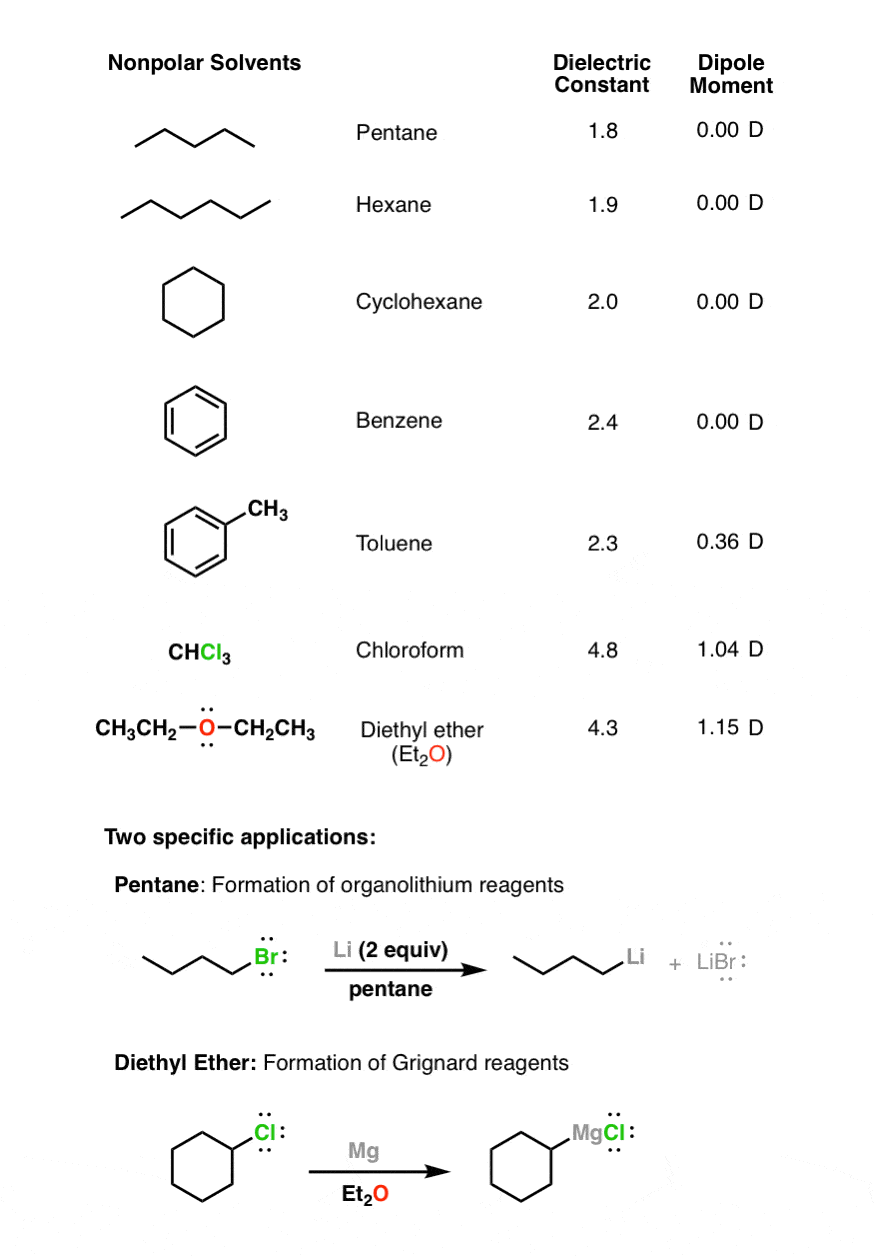
Hence ClF3 is polar in nature. 2. Dipole moment. This is an accurate way to determine whether ClF3 is polar or non-polar. If the molecule has some net dipole moment then that molecule is polar in nature. The higher the dipole moment of the molecule greater is the polarity strength of that molecule.
No, the glucose is too big to pass through the channel in the diagram on the left 21. Explain in detail what happened that allowed the glucose molecules to pass through. A hormone molecule attached to the top of the protein and caused it to change shape; the channel became larger and.
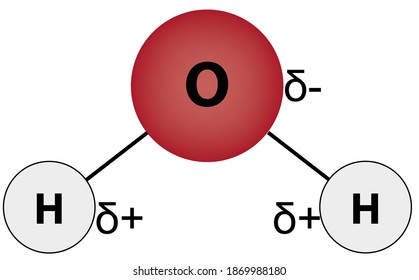
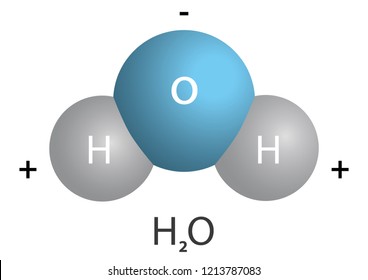
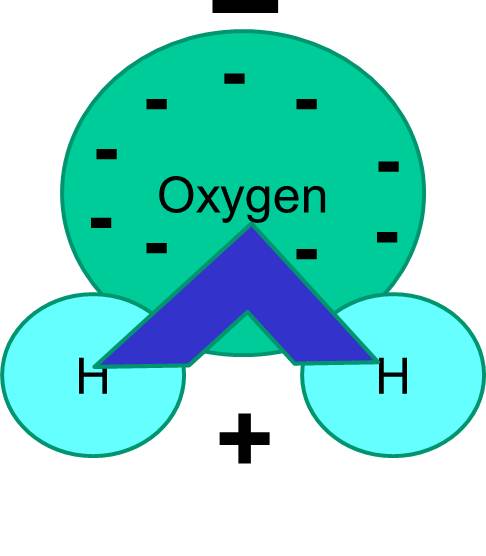
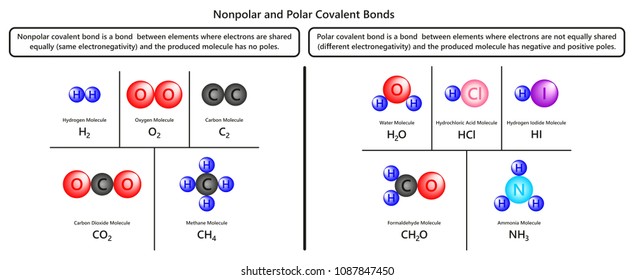
A polar molecule has two poles, which are the positive pole and the negative pole. This comes about because the different atoms in the molecule have different degrees of attraction for electrons.
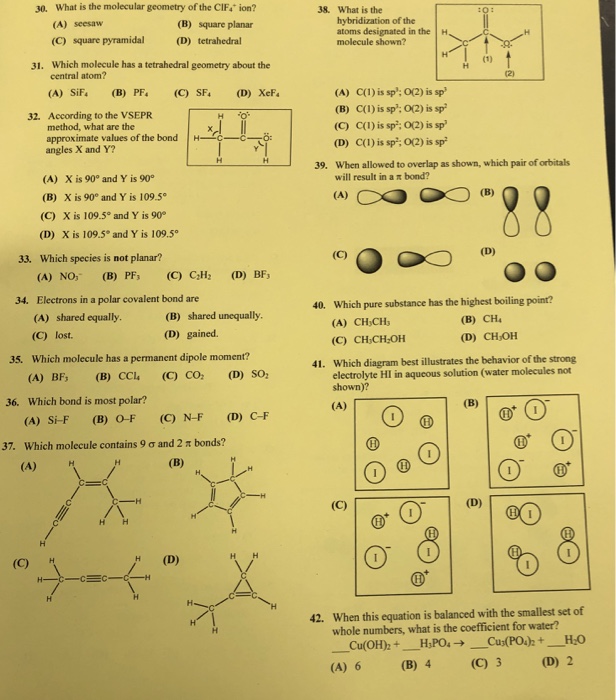
___ 11) Which structural formula represents a polar molecule? 1) 2) 3) 4) ___ 12) A diamond consists of covalently bonded carbon atoms. The diamond is an example of
13. Which formula represents a nonpolar molecule containing polar covalent bonds? 1. 1. 2. 14. 1 at is the chemical fonnula for lead(IV) oxide? b02 2. Pb04 3. Pb20 4. Pb40 15. In the formulaXS04, the symbol X could represent the element 16. 4. Na.ch compound has the strongest hydrogen bonding at STP? 2. 20 3. 4. H2Te 17.
Which diagram best represents a polar molecule? Look at paper. Which electron dot structure formula represents a substance that contains a nonpolar covalent bond. Look at paper. The shape and bonding in a diatomic bromine molecule are best described as. Symmetrical and non-polar.
Which diagram best represents a polar molecule B) NaCl. 19. Base your answer to the following question on the information below and on your knowledge of chemistry. A sample of seawater is analyzed. The table below gives the concentration of some ions in the sample. Concentration of Some Ions in a Seawater Sample Ion
Which formula represents a nonpolar molecule containing polar covalent bonds? 1. H O 3. NH2 3 2. CCl 4. H4 2 29. When an atom of chlorine forms an ionic bond with an atom of sodium, the atom of chlorine 1. loses an electron 3. becomes an ion with a smaller radius than the atom of chlorine
14) The bond between hydrogen and oxygen in a water molecule is classified as a) covalent and nonpolar c) ionic and polar b) ionic and nonpolar d) covalent and polar 15) Which is a nonpolar molecule containing a nonpolar covalent bond? a) I 2 b) CO 2 c) NH 3 d) H 2O 16) Which diagram best represents a polar covalent molecule?
For cations subtract one electron for each unit of positive charge. 1h2o 2ccl4 3nh3 4h2 14which formula represents a nonpolar molecule containing. A i 2 b co 2 c nh 3 d h 2o 16 which diagram best represents a polar covalent molecule. 1h2 2h2o 3co2 4ccl4 12which formula represents a polar molecule.
Which image, F 2 or HF, do you believe represents a asymmetric distribution of electron density? The image of HF shows a symmetric distribution of electrons. In the image of HF what do you think the color blue and color red represent? H-F is a polar covalent molecule due to the dfference in electronegativity between hydrogen and fluorine.
This diagram of CH 4 illustrates the standard convention of displaying a three-dimensional molecule on a two-dimensional surface. The straight lines are in the plane of the page, the solid wedged line is coming out of the plane toward the reader, and the dashed wedged line is going out of the plane away from the reader.
answer choices. The carbon-to-carbon bond on the left is stronger because it is a double bond. The carbon-to-carbon bonds are the same strength because the C-C-C bond angle is 180°. The carbon-to-carbon bonds are the same strength because they are both bonds between carbon atoms.
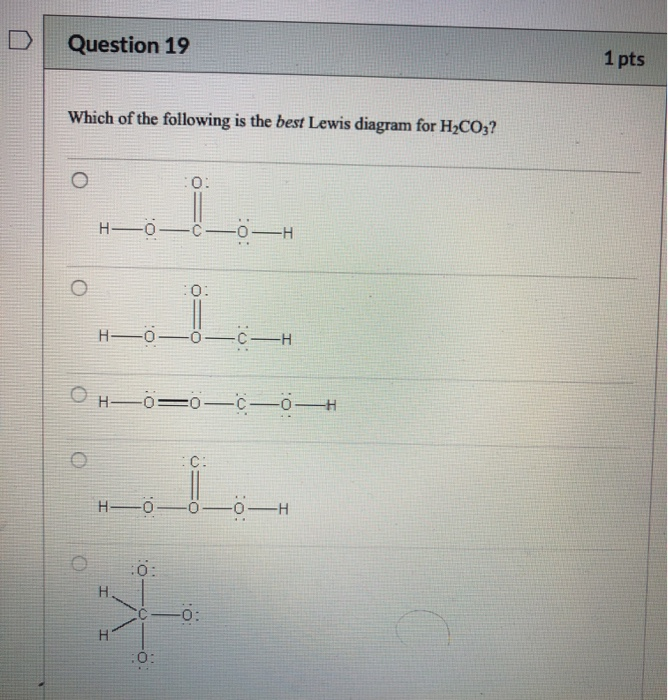
Which electron dot diagram best represents a compound that contains both ionic and covalent bonds? O Ca +2. [ O S O ]. Explain, in terms of electronegativity difference, why the bond between hydrogen and oxygen in a water molecule is more polar than the bond between hydrogen and nitrogen in an ammonia molecule.
16 Questions Show answers. Q. What kind of bond do you have: F and Cl. Q. Which of the following elements has the weakest attraction for electrons in a chemical bond? Q. The molecule shown in the diagram can best be classified as a. Q. The polarity of a bond between two elements can be best determined by.












0 Response to "39 Which Diagram Best Represents A Polar Molecule"
Post a Comment