45 o2 lewis dot diagram
Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. H2o2 Dot Diagram - Wiring Diagrams H2o2 Dot Diagram. The chemical name for H2 O2 is hydrogen peroxide. Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in. Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around the.
O2 Lewis Structure: How to Draw the Dot Structure for O2 ... Drawing the Lewis Structure for O 2 Viewing Notes: For the Lewis Structure for O 2 you're going to need a double bond in order to for each Oxygen atom to have an octet. That's the only way you can make it work with the twelve valence electrons available. Be sure that you don't use more than the tweleve valence electrons available.
O2 lewis dot diagram
patapum.to.itFlour Mill Rye [4MH368] Rye flour contains gluten, but not a lot, so it must be used in conjuction with other. 00 Quick Shop. In addition, railroads made it cheaper to ship wheat to Minneapolis/St. Lewis Structures: Dot Symbols, Diagrams, Examples - Embibe Steps to Draw Lewis Structure The steps to draw the Lewis structures of various types of compounds are given below: Lewis Structure of oxygen \ (\left ( { { {\rm {O}}_ {\rm {2}}}} \right)\) Oxygen belongs to group \ (16\) of the Periodic Table. Hence, oxygen has \ (6\) valence electrons. Step 1 - Calculating the total valence electrons. 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
O2 lewis dot diagram. CO2 Lewis Structure - Lewis Dot Structure | Chem Helps CO2 Lewis Structure can be drawn with 4-step technique. When drawing the Lewis structure of the carbon dioxide molecule, the carbon and an unpaired electron of oxygen share with each other. As a result, a single covalent bond between carbon and oxygen occurs. However, in this case, carbon and oxygen cannot complete the octet. Lewis Structure Of O3 - ozone o3 lewis dot structure ... Lewis Structure Of O3 - 13 images - o2 lewis dot structure cloudshareinfo, how to determine the lewis dot structure of o2 quora, molecular modeling and lewis dot structures, lewis dot diagram for o3, Lewis Dot Diagram - Organic Chemistry | Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE. How to Draw the Lewis Dot Structure for O 2- (Oxide ion ... A step-by-step explanation of how to draw the O2- Lewis Dot Structure.For the O 2- structure use the periodic table to find the total number of valence elect...
SO2 Lewis Structure - Lewis Dot Structure | Chem Helps Lewis structure can be drawn if you know valence electrons of each atom that create the molecule. 1 sulfur atom and 2 oxygen atoms create the SO2 molecule. There are 6 valence electrons in oxygen atom. There are 2 oxygen atoms in SO2 molecule which means that there are 12 valence electrons coming from 2 oxygen atoms. Sulfur valence electrons are 6. How to Draw the Lewis Dot Structure for O2 : Diatomic ... O2 is also called Diatomic Oxygen. ----- Steps to Write Lewis Structure for compounds like O2 ----- 1. Find the total valence electrons for the O2 molecule. 2. Put the least electronegative atom in... OF2 Lewis Structure, Molecular Geometry, Hybridization ... The central atom in an oxygen difluoride molecule is Oxygen. Step 3: Sketch the Skeletal Diagram of the Molecule. In Lewis Structure, we use atomic symbols like C for carbon, H for hydrogen to represent the constituent atoms, and electron dot notation to represent the valence shell electrons. Let us look at the below skeletal sketch: O2 (Oxygen) Lewis Dot Structure - Science Trends Dioxygen (O2) is used in cellular respiration by many living organisms, used to create energy along with sugars. How To Interpret A Lewis Structure Lewis structures are diagrams that represent atoms and the bonds between them. The letters represent the atoms found within the molecule, with specific letters representing different elements.
SiO2 Lewis Structure (Silicon Dioxide) - YouTube Hi Guys, for today's video, we will help you determine the Lewis Structure of Silicon Dioxide. It consists of one Silicon and two Oxygen atoms. And by follow... Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Lewis Electron Dot Diagrams | Introductory Chemistry - 1st ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Lewis dot diagram for oxygen? - Answers Add your answer: Earn + 20 pts. Q: Lewis dot diagram for oxygen. Write your answer... Submit. People also asked.
So2 Lewis Dot Diagram - schematron.org To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too. You know that both the Sulphur and Oxygen has six valence electrons each. 70 More Lewis Dot Structures. S does not follow the octet rule.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures.
Lewis Dot of Oxygen O2 - Kentchemistry.com Lewis Dot of Oxygen O 2 Back 70 More Lewis Dot Structures Element number 8 and a member of the Chalcogen Family or Group 16 of the periodic table. At STP, two atoms of the element bind to form dioxygen, a colorless, odorless, tasteless diatomic gas with the formula O 2. from l
Lewis Dot Diagram For O2 - schematron.org Now, this is only one way we can draw the electron dot diagram for Oxygen. So as you may of remember from Chemistry class, before it can pair up on any other . Answer to (a) Construct a Lewis structure for O2 in which each atom achieves an octet of electrons. (b) Explain (b) Explain why it is necessary to form a double bond in the Lewis structure.
F2O or OF2 lewis dot structure, molecular ... - Topblogtenz Place Oxygen at the center in the lewis diagram and fluorine spaced evenly around it. 3. Connect oxygen and fluorine with a single bond Now in this step, we will start to draw the Lewis structure of F2O by simply connect a fluorine atom with a central atom with a single bond. By looking at the above structure, we see 2 single bonds are used.
Lewis Dot Diagrams | 2022 AP Chem Unit 2 Study Guide ... Since there are 12 total and the octet rule is fulfilled on both atoms, this is the lewis dot structure of O2. 💡Try drawing the lewis dot structure of N2 (answer at the end of this guide). b) CS2. Looking at the periodic table, we know that C has 4 v.e. and S has 6 v.e.. This accounts for a total of 16 valence electrons.
techiescientist.com › sio2-lewis-structureSiO2 Lewis Structure, Molecular Geometry, Hybridization, and ... May 07, 2022 · Since there is no lone pair on the central atom of the SiO2 Lewis dot diagram, the bond angle is 180 degrees. This means there is no impact on the bond angle since there is no repulsion between the lone and bond pair. According to VSEPR theory, “The geometry around an atom with just two bonds and no unshared electrons is a straight line,”
SiO2 Lewis Structure| Step By Step Construction - What's ... SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2.The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure.. Step-1: Count the valence electrons of atoms For the SiO 2 Lewis structure, we need to figure out the number of valence ...
Oxygen (O2) Molecule Lewis Structure Oxygen (O 2) Molecule Lewis Structure Oxygen is a diatomic molecule and contains only two oxygen atoms. In the lewis structure of O 2 molecule, a double bond is located between oxygen atoms and each oxygen atom has two lone pairs in their valence shells. There are many things to learn when we draw the O 2 lewis structure. O 2 lewis structure
O2 Lewis Structure, Molecular Geometry, and Hybridization ... The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule.
CO2 Lewis Structure | Lewis Dot Structure For Molecules ... So, the exact NO2-1 lewis dot structure is: SO3 2- Lewis Structure SO3 2- lewis structure, first, we calculate Q. Q = Valance electron of all-atom + no of -ve charge - no of +ve charge Q = (24 + 2 - 0) Q = 26 B.P e - = 2 × no of bonds
Lewis Electron Dot Structures: Steps, Examples and Limitations Lewis Electron Dot Structure of the molecule: CO (carbon monoxide) The carbon atom has a valency of 4. Hence it needs 4 more to complete the octet. The bonding oxygen atom needs two more to fill its octet. So to satisfy the octet structure we get a triple bond between C and O atoms. Lewis Electron Dot Structure for the molecule: HCOOH (Formic Acid)
en.wikipedia.org › wiki › Radical_(chemistry)Radical (chemistry) - Wikipedia The hydroxyl radical, Lewis structure shown, contains one unpaired electron In chemistry , a free radical is an atom , molecule , or ion that has at least one unpaired valence electron . [1] [2] With some exceptions, these unpaired electrons make radicals highly chemically reactive .
quizlet.com › 243872019 › mastering-chemistry-ch8Mastering Chemistry Ch.8 Flashcards | Quizlet How many nonbonding electron pairs are there in the Lewis structure of the peroxide ion, O22−? The peroxide ion, O22−, contains 14 electrons in its Lewis structure of which there are 6 pairs on nonbonding electrons and 1 pair of bonding electrons.
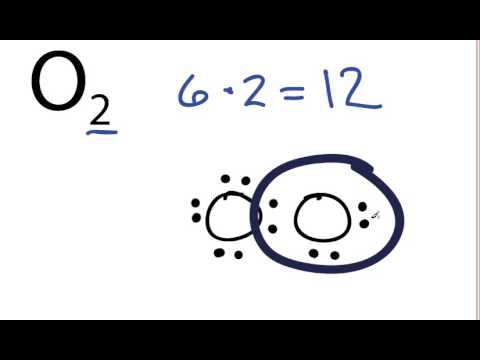







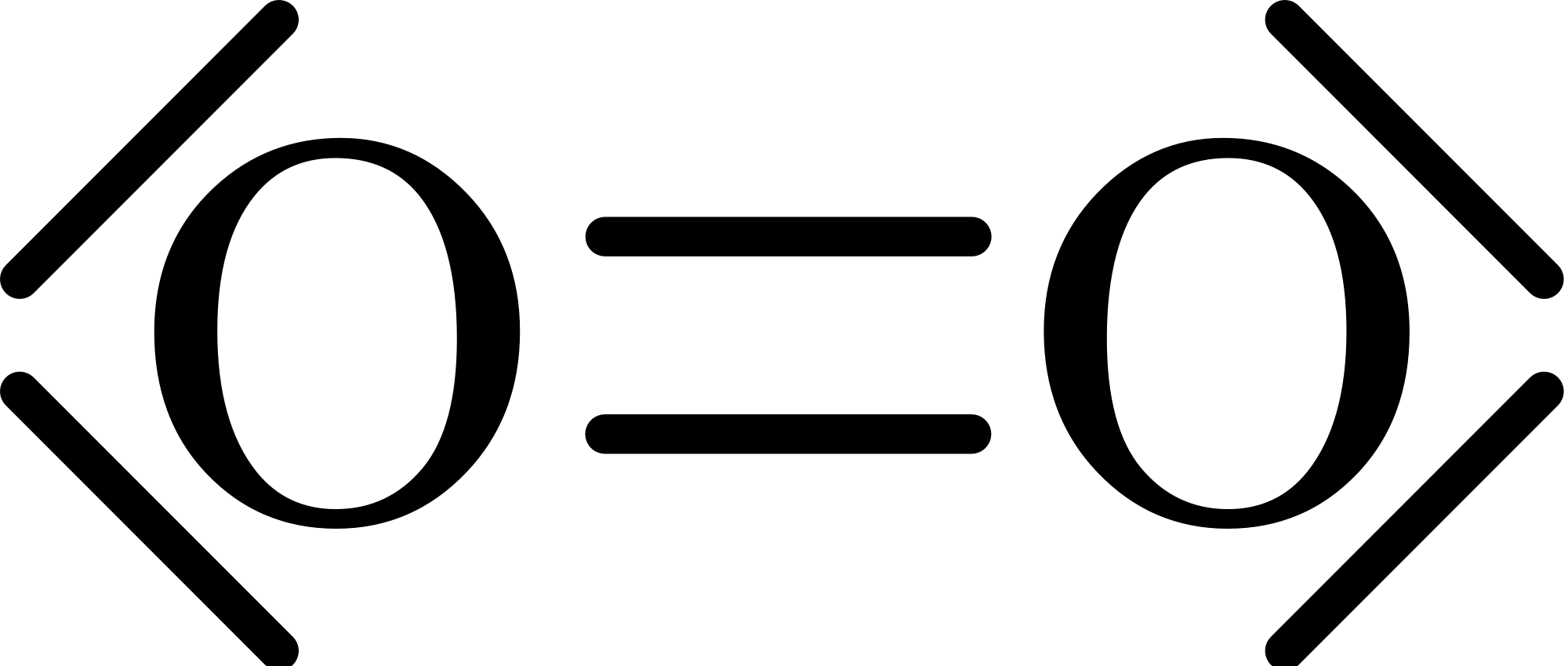
0 Response to "45 o2 lewis dot diagram"
Post a Comment