41 Sn Pb Phase Diagram
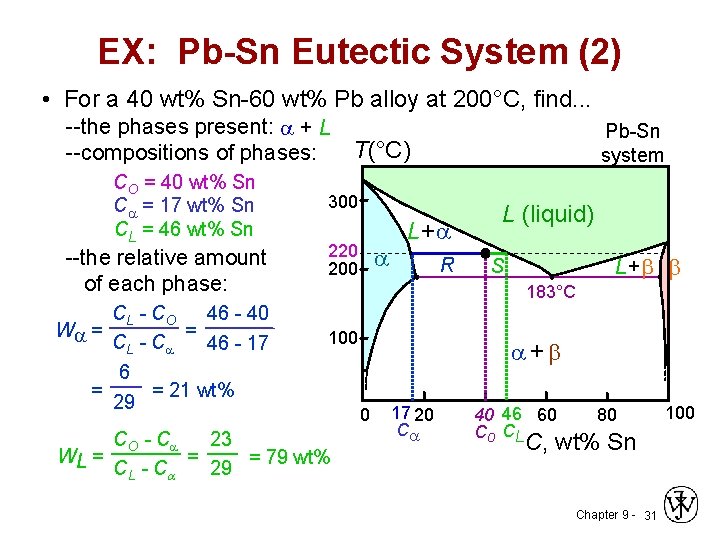
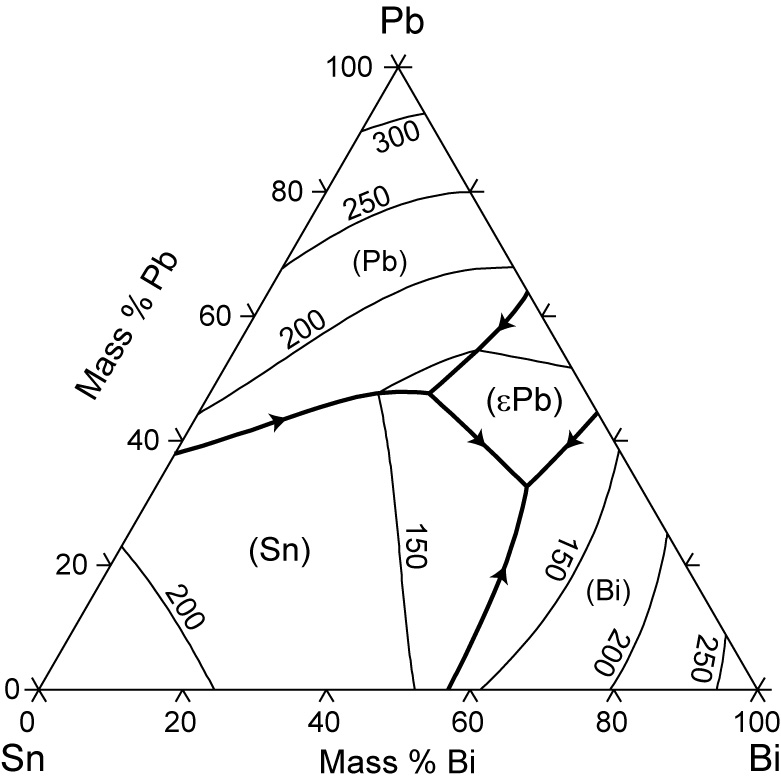
Created Date: 11/3/2015 10:28:58 AM The tin-bismuth phase diagram Composition of the Bi-Sn soldier: 57wt% Bi Questions: 1. Give the names for the single phases in the Sn-Bi system 2. To fíll the name for each phase region 3. The eutectic. Pb-Sn system EX: Pb-Sn Eutectic System (1) a+ b--compositions of phases: C O = 40 wt% Sn--the relative amount of each phase: 150 40 Co 11...
This problem has been solved! At 100 C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown below. Round to the nearest whole number. Who are the experts? Experts are tested by Chegg as specialists in their subject area.
Sn pb phase diagram
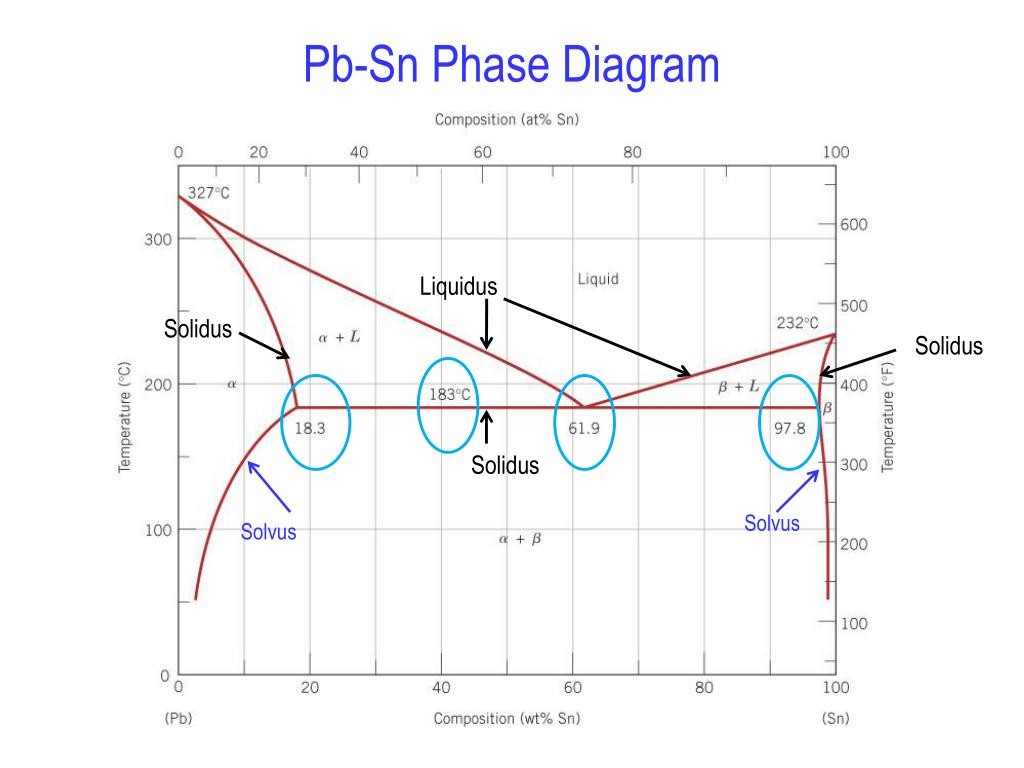
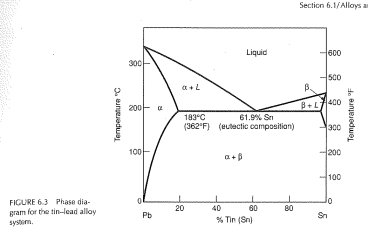
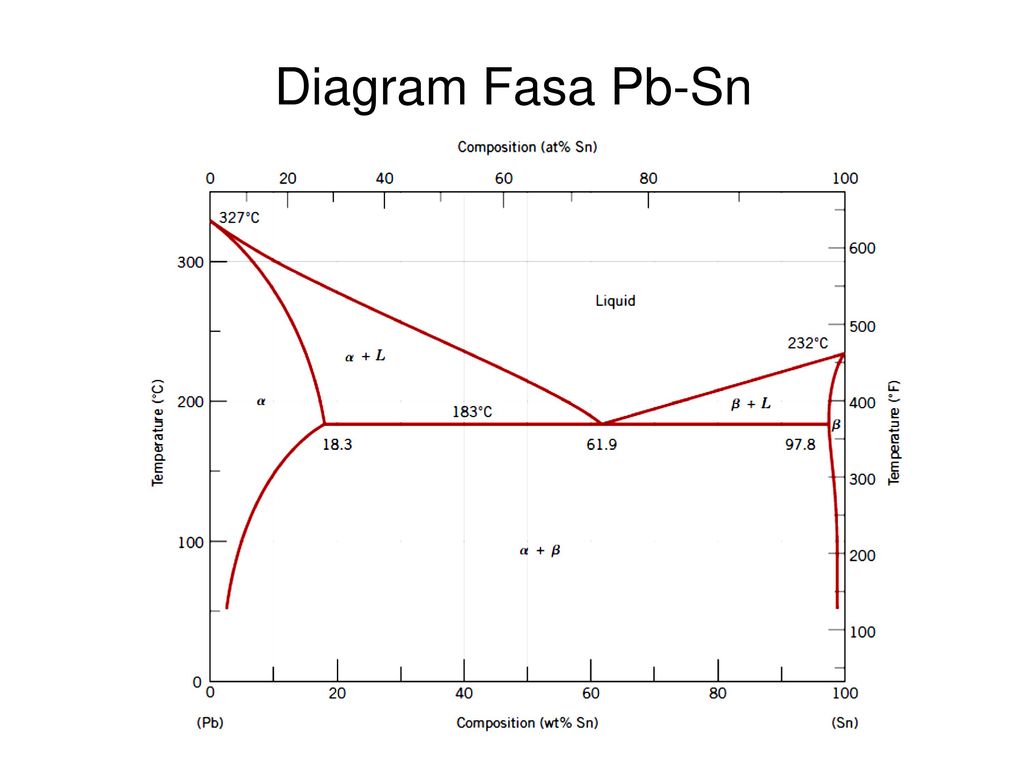
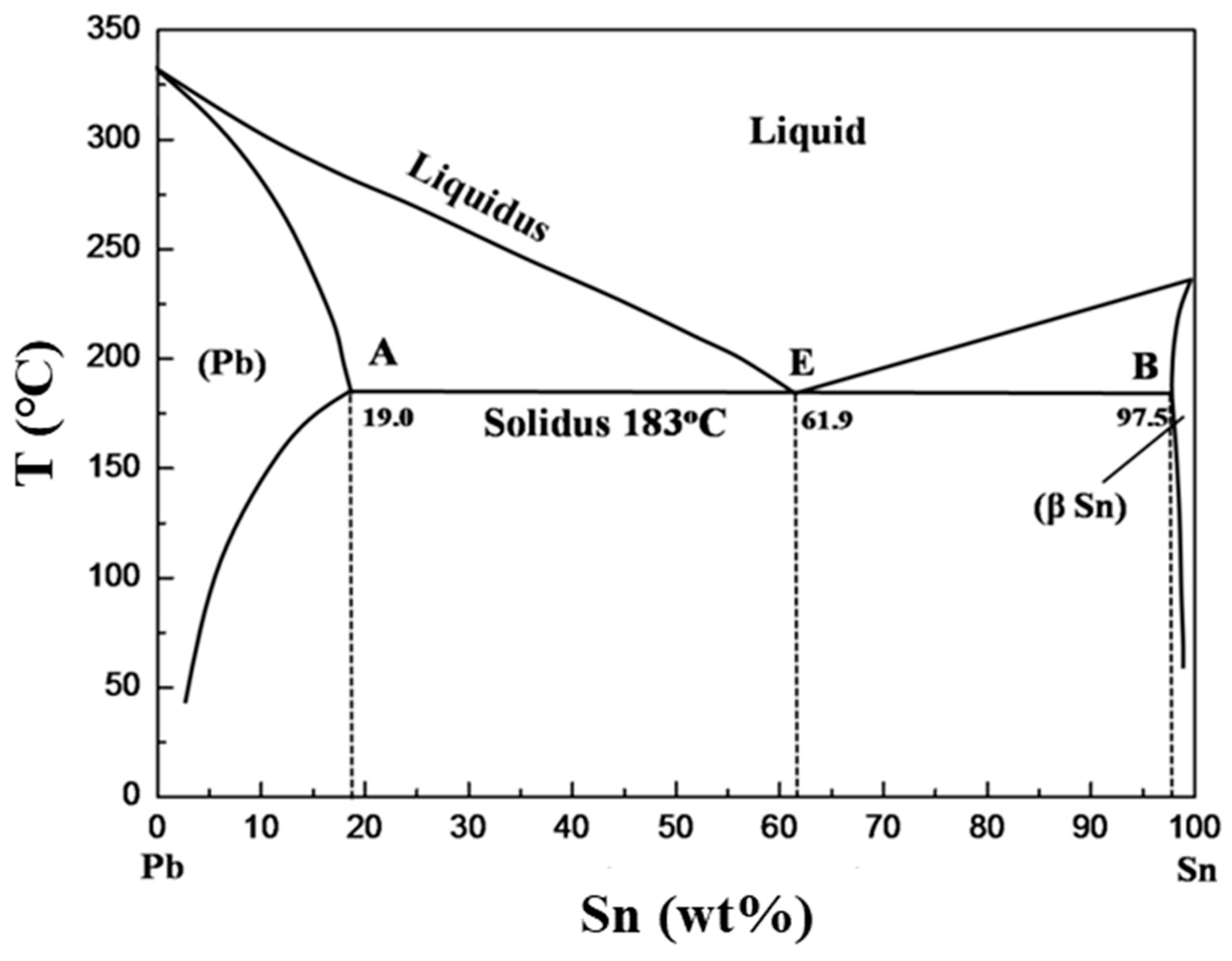
Pb-Sn phase diagram β phase: solid solution of Pb in tetragonal Sn α phase: solid solution of Sn in fcc Pb Liquid Pb (Fcc) Sn (Tetra) 0 50 100 150 200 250 300 350 0 10 20 30 40 50 60 70 80 90 100 T emperature Wt% The Pb-Sn system is characteristic of a valley in the middle. Such system is known as the Eutectic system. Similarly, P-x VLE phase diagram of Pb-Sn system at the temperature range from 1073 K to 1473 K are calculated.Fig. 3(b) shows that the lower distillation temperature was supplied, the higher vacuum degree was needed to afford. Table 4 shows the comparison of contents of x Pb and y Pb for Pb in crude Sn between experiment and prediction. The results show not satisfactory the agreement of Pb. Sn-Pb Eutectic System: i. For a 40% Sn – 60% Cu alloy at 150°C. Calculate composition of phase present. ii. Amount of each phase present in terms of main fractions and volume fractions. Given density of Pb and Sn are 11.23 kg/cm 2 and 7.24 g/cm 3. Binary Phase Diagram of Type-III:
Sn pb phase diagram. โลหะผสมมีส่วนผสม 75 wt% Sn - 25 wt% Pb ณ อุณหภูมิ 150oC จงหาว่า (a) มีเฟส (phase) ใดปรากฎบ้าง (b) เฟสที่ปรากฎนั นมีส่วนผสมทางเคมีเท่าใหร่ Figure 6.4 Ideal cooling curves and the construction of phase diagram 6 -time diagram of a Pb-Sn alloy P = 3, C = 2, therefore F = 0. The temperature should 6.5. s. Fig. , for example, those. 7 III. Measurement Experimental setup The data were recorded using a computer controlled data acquisition system. The alloys have been melt Eutectic 1. We have examined isomorphous phase diagrams, and used the example of the Cu-Ni phase diagram. In this module we will examine eutectic phase diagrams. A eutectic system has two components, and they have limited solubility. Upon cooling, the liquid will transform into two mixed solid phases. We will use the Pb-Sn phase diagram as an. suggested as possible alternatives to Pb-Sn solders. 19 Figure 6 shows the effect of the addition of Ag, Bi, Sb, or Zn on the phase constitution of Sn-20mass%In alloys. a Fig. 3. Isothermal section diagrams of the Sn-In-Bi system at (a) 100°C and (b) 200°C. a Fig. 4. Isothermal section diagrams of the Sn-In-Sb system at (a) 100°C and (b) 200.
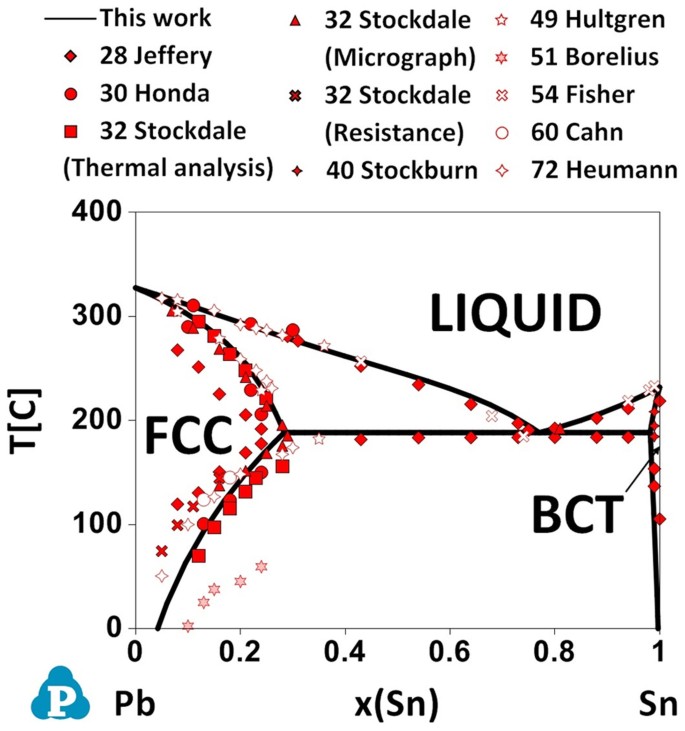
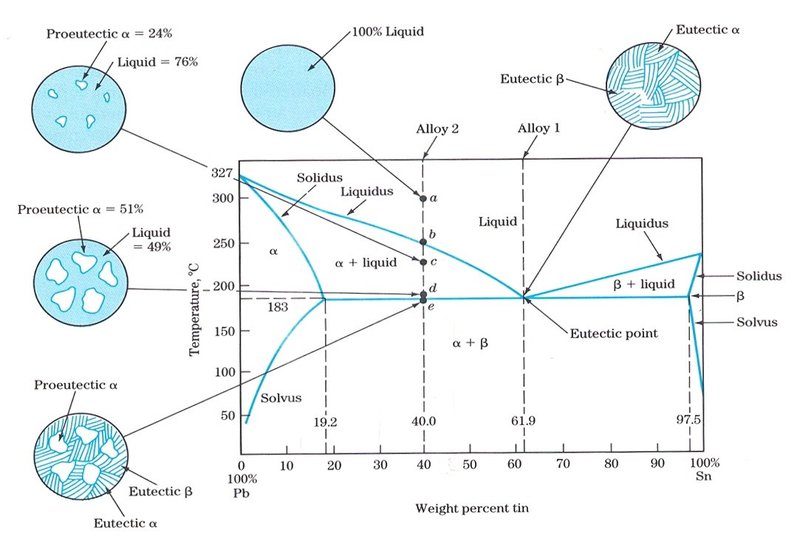
金属やセラミックスの状態図、エンリンガム図などの情報を提供しています。 一部、不確かなものもありますので、自己. 9.38 On the basis of the photomicrograph (i.e., the relative amounts of the microconstituents) for the lead- tin alloy shown in Figure 9.17 and the Pb-Sn phase diagram (Figure 9.8), estimate the composition of the alloy, and then compare this estimate with the composition given in the figure legend of Figure 9.17. Bi-Sn Eutectic Pb-Sn Eutectic at 960C M as s F r a t i o n o f B i M s s F r c t o n o f P b Bi Mass Fraction of Sn Sn t c / 0 C prism is a two-component temperature-composition phase diagram with Pb Triple Eutectic 3-Dimensional Depiction of Temperature-Composition Phase Diagram of Bismuth, Tin, and Lead at 1atm. The diagram has been simplified by The thermodynamic description of Sn-Bi-Pb from Yoon and Lee, Calphad 22 (1998) 167 was used for the present calculations. The liquid concentration paths for the alloys, Sn-.04Bi-.06Pb and Sn-.49Bi-.06Pb, are shown together with the liquidus projection of Sn-Bi-Pb system. The (Sn) phase is the primary phase in all cases.
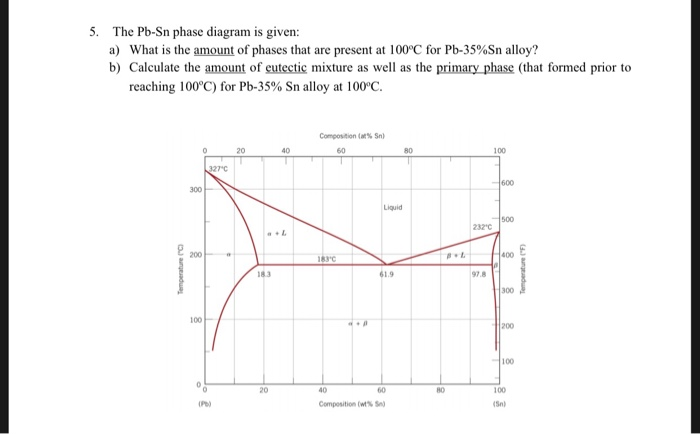
(b) That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown below; the point labeled “B” represents the 75 wt% Sn-25 wt% Pb composition at 175°C. As may be noted, point B lies within the α + β phase field. A tie line has been constructed at 175°C; its intersection Download scientific diagram | Pb-Sn phase diagram. from publication: Partial melting of a Pb-Sn mushy layer due to heating from above, and implications for regional melting of Earth's. 金属やセラミックスの状態図、エンリンガム図などの情報を提供しています。 一部、不確かなものもありますので、自己. Phase Struktur-bericht Symbol Common Names Prototype Spacegroup Model * Liquid: n/a: L, L 1, L 2: n/a: n/a (Ag,Pb,Sn) 1 : Fcc: A1 (Ag), (Pb) Cu: Fm-3m (Ag,Pb,Sn) 1 (Va) 1: Hcp: A3 (zeta Ag), (epsilon Pb) Mg: P6 3 /mmc (Ag,Pb,Sn) 1 (Va) 0.5: Bct: A5 (Sn), (beta Sn) beta Sn: I4 1 /amd (Ag,Pb,Sn) 1 : Ag 3 Sn: D0alpha: epsilon: beta Cu 3 Ti: Pmmn.
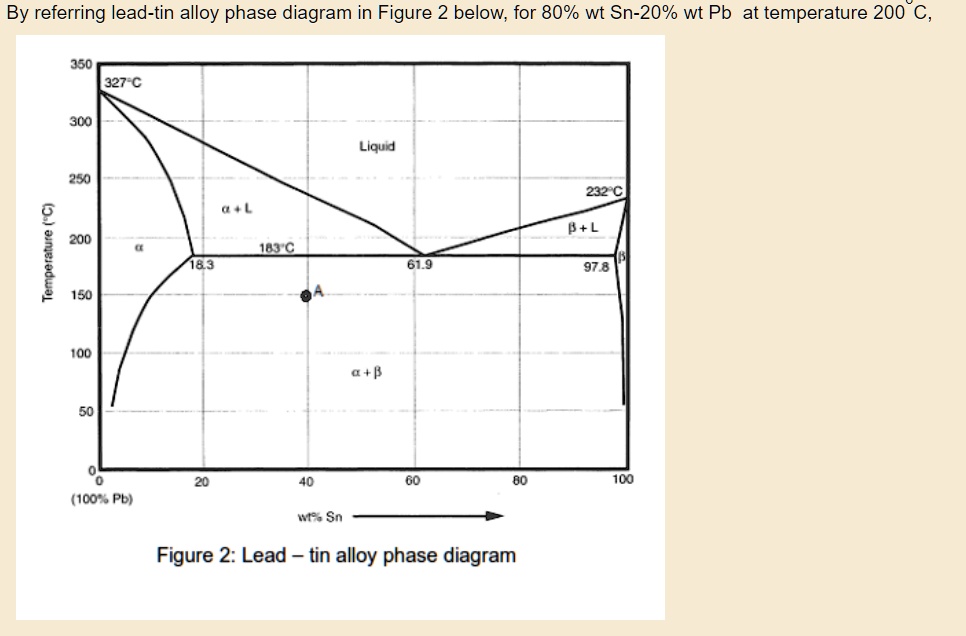
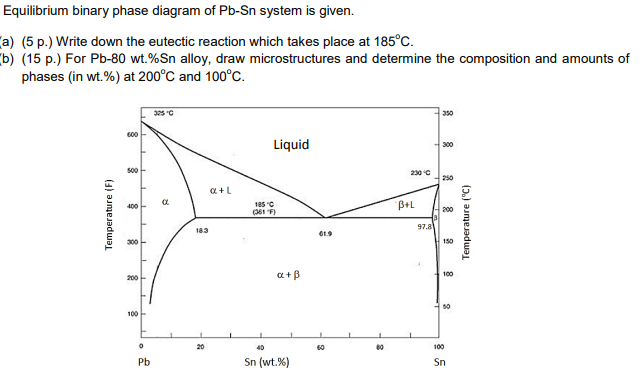
Figure 1 shows the Pb-Sn phase diagram and the composition for present experiments, in which it is very clear that the eutectic temperature is 183 • C, and the liquid-solid transition.
Pb-Sn phase diagram β phase: solid solution of Pb in tetragonal Sn α phase: solid solution of Sn in fcc Pb Liquid Pb (Fcc) Sn (Tetra) 0 50 100 150 200 250 300 350 0 10 20 30 40 50 60 70 80 90 100 T emperature Wt% The Pb-Sn system is characteristic of a valley in the middle. Such system is known as the Eutectic system. The central point is the.
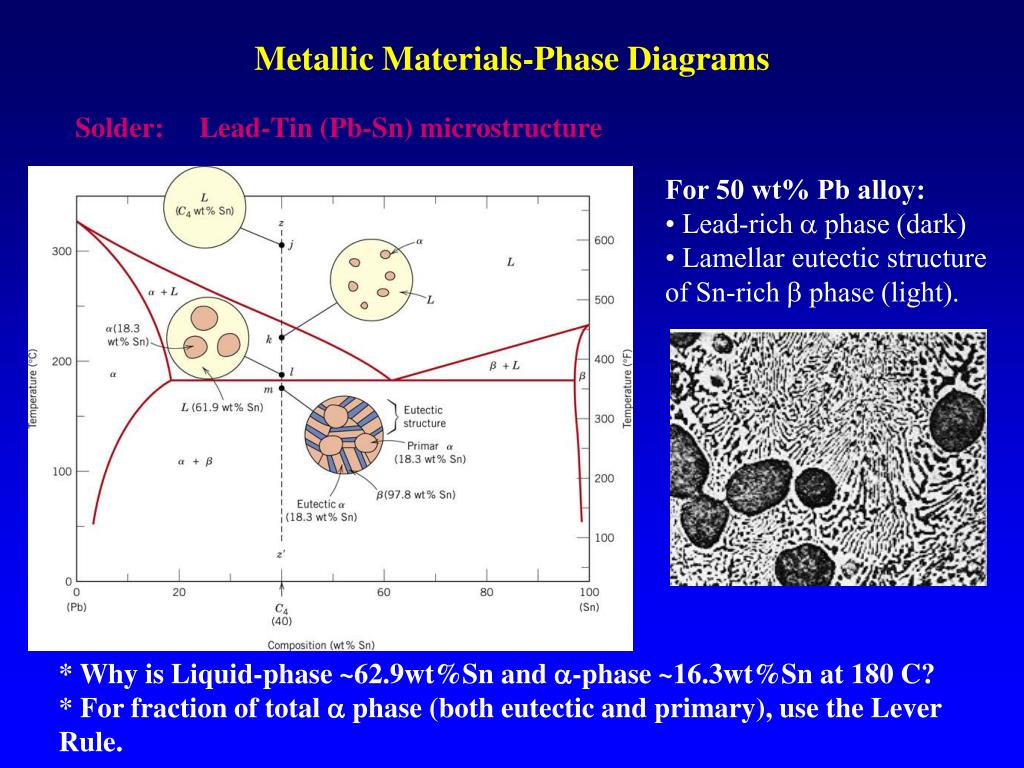
Sugar/Water Phase Diagram S u g a r T e m p e r a t u r e. the Pb-Sn system during solidification at the eutectic composition. Compositions of α and β phases are very different. Solidification involves redistribution of Pb and Sn atoms by atomic diffusion. Pb-rich
Important: This is a simplified version of the real tin-lead phase diagram.In particular, it ignores the formation of solid solutions of tin and lead. You will find the correct diagram on this NIST web page.Beware that on that page, the tin-lead axis is reversed from the one I have drawn above - in other words 100% lead is on the right rather than the left.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators.
This video explains the Pb-Sn phase diagramFor further reading: https://www.physicsforums /threads/sn-pb-phase-diagram.281790/
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Phase Diagram Resources from ASM International. Alloy phase diagrams and crystal structure data are used by metallurgists, materials engineers, and materials scientists to develop new alloys for specific applications; fabricate these alloys into useful configurations; design and control heat treatment procedures for alloys that will produce the required mechanical, physical, and chemical.
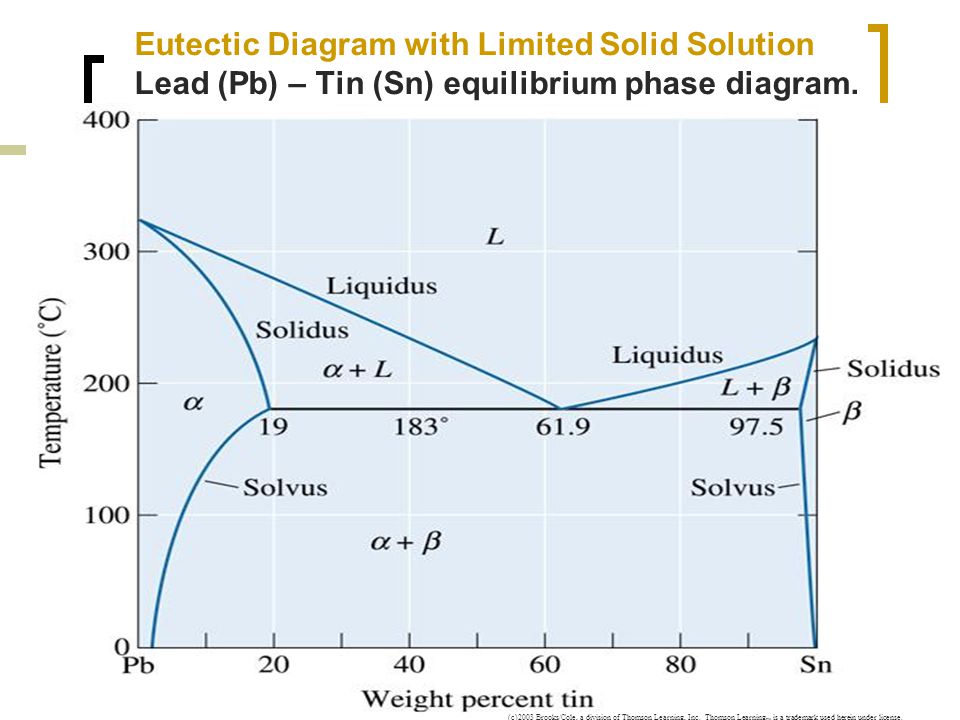
Below is the binary phase diagram for Pb-Sn. At 46.5 wt% Sn, what is the total weight fraction of a (alpha) phase just below the eutectic solidification temperature? 400 |327°C 300 Pb-80%Sn Pb-61.9%Sn 231°C Temperature,[°C] 200 F (Pb) 183°C 19 61.9 97.5 Sn 100 0 1 1 1 0 10 20 50 70 80 90 100 30 40 60 Pb-Sn[wt%] 0.65
A phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically-distinct phases; or to show what phases are present in the material system at various T, p, and compositions. • Example: cooling 60%Pb - 40%Sn system
Below is the phase diagram of the lead (Pb)-tin (Sn) system. In this section, the Pb-Sn phase diagram will be discussed in detail, but first there a few basic components of phase diagrams to discuss. First there are the axes. The vertical axis shows temperature, while the horizontal axis shows the concentration of some species.
Sn-Bi-Pb equilibrium phase diagram. For the Lever calcula-tion, complete mixing in the liquid and complete diffusion in † In this paper, the percentage presents mass fraction, which is the same as weight fraction. ‡ The changeover to Pb-free solder technology requires changes in pre-tinning operations as well as solder.
Alloy Phase Diagrams 9 (1988) 144-152. H. Ohtani, K. Okuda and K. Ishida, J. Phase Equilibria 16 (1995) 416-429. Calculated Invariant Equilibria. Reaction. Phase. Mass % Pb. Mass % Sn. L -> (Pb) + (Sn) 182.2 o C.
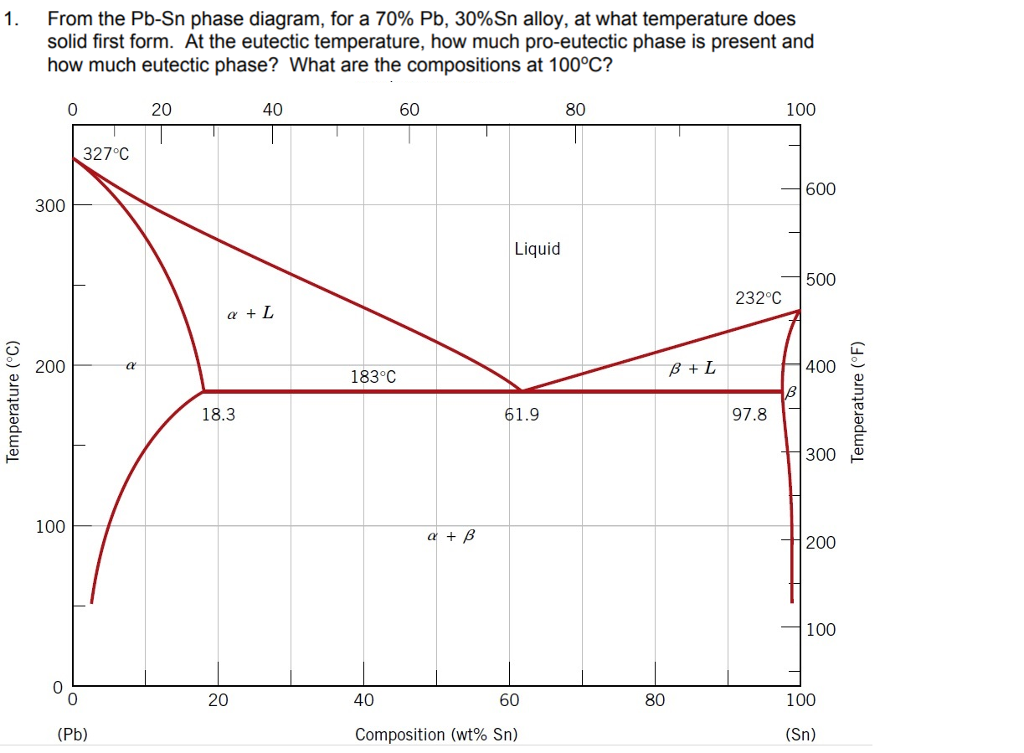
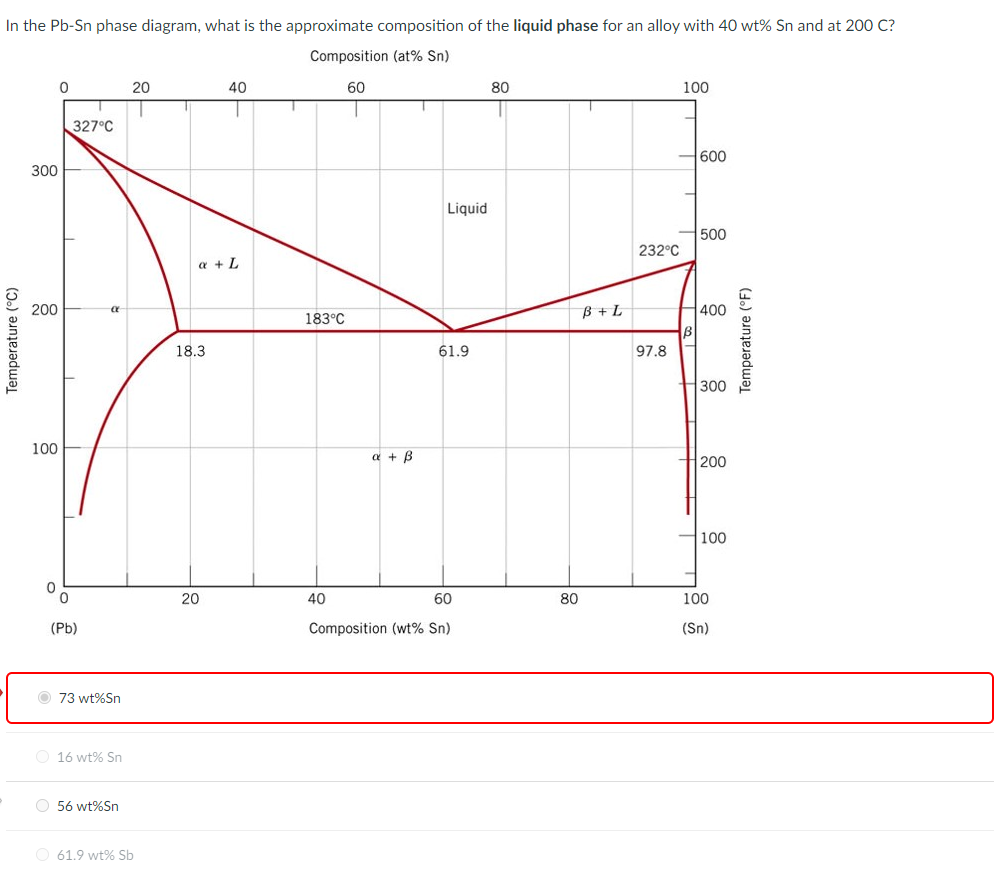
The Attempt at a Solution. It is probably an easy question, but I thought it is a good idea to consult first. a) At 183 C, the first liquid phase forms. b) We can draw a tie line and the point intersects with the liquidus line, gives us the composition of liquid. It is 61.9 wt % Sn. c) It is around 250 C. Because phase diagram is on liquidus line.
Pb-Sn phase diagram β phase: solid solution of Pb in tetragonal Sn α phase: solid solution of Sn in fcc Pb Liquid Pb (Fcc) Sn (Tetra) 0 50 100 150 200 250 300 350 0 10 20 30 40 50 60 70 80 90 100 T emperature Wt% The Pb-Sn system is characteristic of a valley in the middle. Such system is known as the Eutectic system.
In the Pb-Sn phase diagram Pb is component A and Sn is component B. Microstructural Component. A component of the microstructure that has an identifiable characteristic morphology that developed due to its composition and thermal treatment. Possible microstructural components on the Pb-Sn phase diagram include alpha and beta in the single phase.
Phase Diagram: 5. Calculations (For 200˚C): Liquid phase 60 wt% of Sn and 40 wt% of Pb Weight fraction of liquid phase is 1 6. Phase Diagram: 7. Calculations (For 100˚C): +β phase phase contains 5 wt% of Sn and 95 wt% of Pb β phase contains 98 wt% of Sn and 2 wt% of Pb. 8.
View the full answer. Transcribed image text: Consider the Sn - Pb phase diagram below. - Sn-Pb system T (°C) 300 L (liquid) Lia [+BIB 2001 a 183°C 100 a+B 0 20 40 60 80 100 C, wt% Sn For a 10 wt% Sn-90 wt% Pb alloy at 120°C, what is the approximate mass fraction of phase B? O 70 % O 53 0 36% 5% O None. Previous question Next question.
Similarly, P-x VLE phase diagram of Pb-Sn system at the temperature range from 1073 K to 1473 K are calculated.Fig. 3(b) shows that the lower distillation temperature was supplied, the higher vacuum degree was needed to afford. Table 4 shows the comparison of contents of x Pb and y Pb for Pb in crude Sn between experiment and prediction. The results show not satisfactory the agreement of Pb.
Sn-Pb Eutectic System: i. For a 40% Sn – 60% Cu alloy at 150°C. Calculate composition of phase present. ii. Amount of each phase present in terms of main fractions and volume fractions. Given density of Pb and Sn are 11.23 kg/cm 2 and 7.24 g/cm 3. Binary Phase Diagram of Type-III:
The binary Pb-Sn phase diagram has been studied for over 100 years and is a classic eutectic. Lead (Pb) has an atomic number of 82 and a high density. Its crystal structure is face-centered cubic (fcc). At 50 C, 2% Sn is soluble in Pb and at the eutectic temperature,
The lead-tin phase diagram is shown below. 400 327°C 300 Pb-80%Sn Pb-61.9%Sn 231° Temperature,[°C] 200 F (Pb) 183°C 19 61.9 97.5 Sn 100 0 0 10 20 30 40 50 60 70 80 90 100 Pb-Sn[wt%] Previous question Next question
β phase. Thus, the phase compositions are as follows: Cα = 16 wt% Sn-84 wt% Pb Cβ = 97 wt% Sn-3 wt% Pb (c) The Ag-Cu phase diagram (Figure 9.7) is shown below; the point labeled "C" represents the 55 wt % Ag-45 wt% Cu composition at 900 °C. As may be noted, point C lies within the Liquid phase field.
represented in the portion of the Pb-Sn phase diagram shown below (at point B). Furthermore, the compositions of the phases, as determined from the tie line are Cα = 16 wt% Sn-84 wt% Pb Cβ = 97 wt% Sn-3 wt% Pb Inasmuch as the composition of the alloy C0 = 75 wt% Sn, application of the appropriate lever rule expressions (for
9.38 On the basis of the photomicrograph (i.e., the relative amounts of the microconstituents) for the lead– tin alloy shown in Figure 9.17 and the Pb–Sn phase diagram (Figure 9.8), estimate the composition of the alloy, and then compare this estimate with the composition given in.
















0 Response to "41 Sn Pb Phase Diagram"
Post a Comment