41 Lever Rule Phase Diagram
Lever Rule Solidification. The calculation of the equilibrium solidification according to the lever rule is a one dimensional stepping calculation in which one of the variables, the temperature, is stepped. At each step a single point equilibrium calculation is carried out. The result of the calculation gives phase compositions, phase fractions. The lever rule. If an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever rule to the phase diagram. The lever rule can be explained by considering a simple balance. The composition of the alloy is represented by the fulcrum, and the compositions of the two phases by the ends of a bar.
Lever-Rule Stefan Bringuier March 2, 2014 The essence of the lever-rule is that the fraction of each phase at a given composition is the ratio of the di erences in composition weight percent (or atomic). Let us take for example a complete solubility system such as Si-Ge with two phases and L, shown in Fig. 1. The total number of moles is given.
Lever rule phase diagram
Phase Diagrams Phase Diagrams and Phase Separation Books MF Ashby and DA Jones, Engineering Materials Vol 2, Pergamon. Proportions of α and β given by Lever rule. For c<cA A dissolves B For cB<c B dissolves A cA and cB define solubility limits. F F1 A1 B1 F3 A3 B3 FA FB A cA c cB B. AM Donald 11 Relative abundance's of the two phases are given by the lever rule. Example 8.2 Interpreting a liquid-liquid phase diagram. A mixture of 50 g of hexane (0.59 mol) and 50 g nitobenzene (0.41 mol) was prepared at 290 K. What are the compositions of the phases, and what proportions do they occur? To what temperature must the sample be heated to. Now that we know the compositions of the two phases, we need to find how much of each phase exists at the given temperature. The ratio of the two phases present can be found by using the lever rule.. At first sight the lever rule can appear confusing. It is really invoking the conservation of mass, and can be proved mathematically, as shown below the diagram.
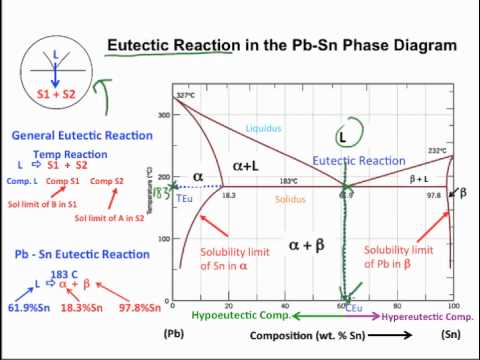
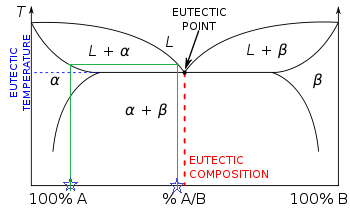
Lever rule phase diagram. lever would balance. • The relationship between the length of connecting or tie lines and the proportions of compositions or minerals described above is a general one and is called the lever rule. The lever rule actually has a much broader application than simply reproducing information that might be read directly from a composition axis. We are given a hypothetical eutectic phase diagram for which Ceutectic = 47 wt% B, Cβ = 92.6 wt% B at the eutectic temperature, and also that Wα' = 0.356 and Wα = 0.693; from this we are asked to determine the composition of the alloy. Let us write lever rule expressions for Wα' and Wα Wα= CβŹ− C0 Cβ−Cα = 92.6 − C0 92.6 − Cα. If the system point is in a two-phase area of the phase diagram, we draw a horizontal tie line of constant temperature (on a temperature–composition phase diagram) or constant pressure (on a pressure–composition phase diagram). The lever rule applies. The lever rule applies to any two-component system existing in a state of two-phase equilibrium. Here, several classroom demonstrations are described to help students understand liquid--liquid coexistence and the lever rule using a set of isobutyric acid + water + dye samples ranging in composition from water + dye to isobutyric acid + dye. The critical temperature of the partially miscible.
Video tutorial illustrating application and derivation of the lever rule in a simple isomorphous binary phase diagram.Video lecture for Introduction to Mater... In thermodynamics, the phase rule is a general principle governing "pVT" systems (that is, systems whose states are completely described by the variables pressure (p), volume (V) and temperature (T)) in thermodynamic equilibrium.If F is the number of degrees of freedom, C is the number of components and P is the number of phases, then = + It was derived by American physicist Josiah Willard. The lever rule is used to calculate the relative amounts of the two phases, which are represented on the bar graph on the right. The compositions of the two phases are indicated by dotted lines on the phase diagram and numerically at the top of the bar graph. Outside the curve, and mix spontaneously to form one layer. Applies the lever rule to a solid-liquid mixture to determine the fraction of each phase in equilibrium and explains the basis for the lever rule. Made by fa...
The lever rule is used to calculate the relative amounts of the two phases, which are shown on the bar graph on the right. The compositions of the two phases are indicated by dotted lines on the phase diagram and numerically at the top of the bar graph. 5.3: The Lever Rule. P-x and T-x diagrams are quite useful, in that information about the compositions and relative amounts of the two phases can be easily extracted. In fact, besides giving a qualitative picture of the phase behavior of fluid mixtures, phase diagrams can also give quantitative information pertaining to the amounts of each. Lever Rule: Index: Shown is a partial binary phase diagram for the Copper-Nickel system with the alloy Cu-35 wt% Ni at the vertical line. At the point, a, the alloy is a liquid. Upon cooling it passes through the two-phase (a + L) zone and then solidifies as a single phase substitutional alloy. Between (b) where the composition line intersects. 1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line The lever rule is a mechanical analogy to the mass balance calculation.
The lever rule is a mathematical procedure that allows us to calculate the fractions, percentages or quantities of the phases present in equilibrium within a binary system. It is not only mathematical, but also quite graphic and assertive, being very useful in physicochemical and engineering calculations. This rule applies to phase diagrams for binary systems, regardless of the.
lever rule cannot be used here, it can be assumed that moving closer to an SPB line will likely increase the amount of the phase, while moving closer. Computer programs that predict phase diagrams can give. a phase diagram in the form of ZPF lines alone. In this case, the lines are labeled instead of the regions.
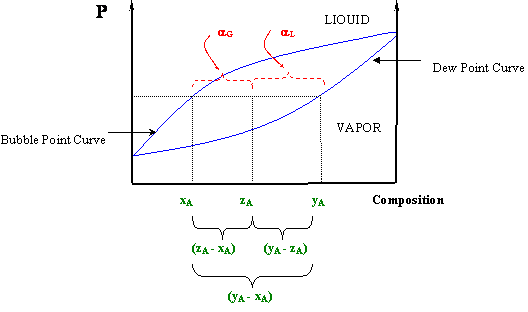
Sometimes it is also known as the "reverse arm rule," because for the calculation of α L (liquid) you use the "arm" within the (y A -x A) segment closest to the vapor, and for the vapor calculation (α G) you use the "arm" closest to the liquid. Figure 5.5: The Lever Rule In a P-x Diagram. At this point you will see clearly why we.
Now that we know the compositions of the two phases, we need to find how much of each phase exists at the given temperature. The ratio of the two phases present can be found by using the lever rule.. At first sight the lever rule can appear confusing. It is really invoking the conservation of mass, and can be proved mathematically, as shown below the diagram.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators.
If the entire length of the tie line mo is taken to represent 100% or the total weight of the two phases present at temperature T, the lever rule may be expressed mathematically as: For such phase diagram, there is one degree of freedom. So, in this case Gibb’s rule converts into
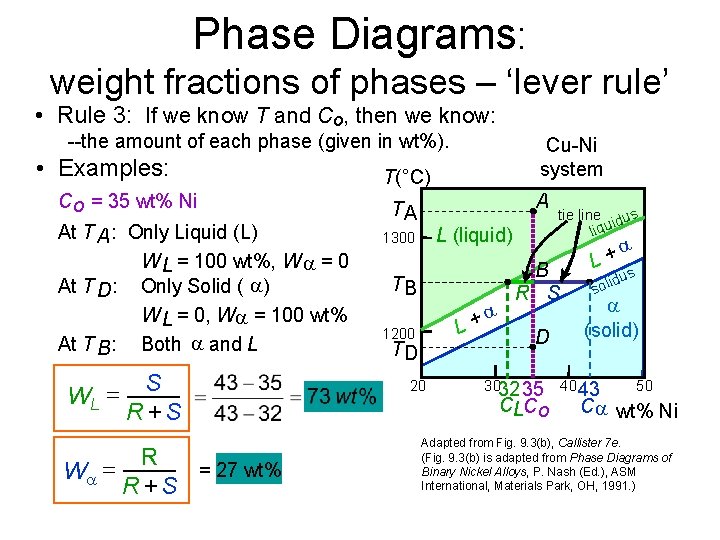
Chapter 8 10 8.5 The Lever Rule The weight percentages of the phases in any 2 phase region can be calculated by using the lever rule Let x be the alloy composition of interest, its mass fraction of B (in A) is Cο Let T be the temperature of interest ⇒at T alloy x consists of a mixture of liquid (with C L-mass fraction of B in liquid) and solid (C S - mass fraction of B in solid phase)



0 Response to "41 Lever Rule Phase Diagram"
Post a Comment