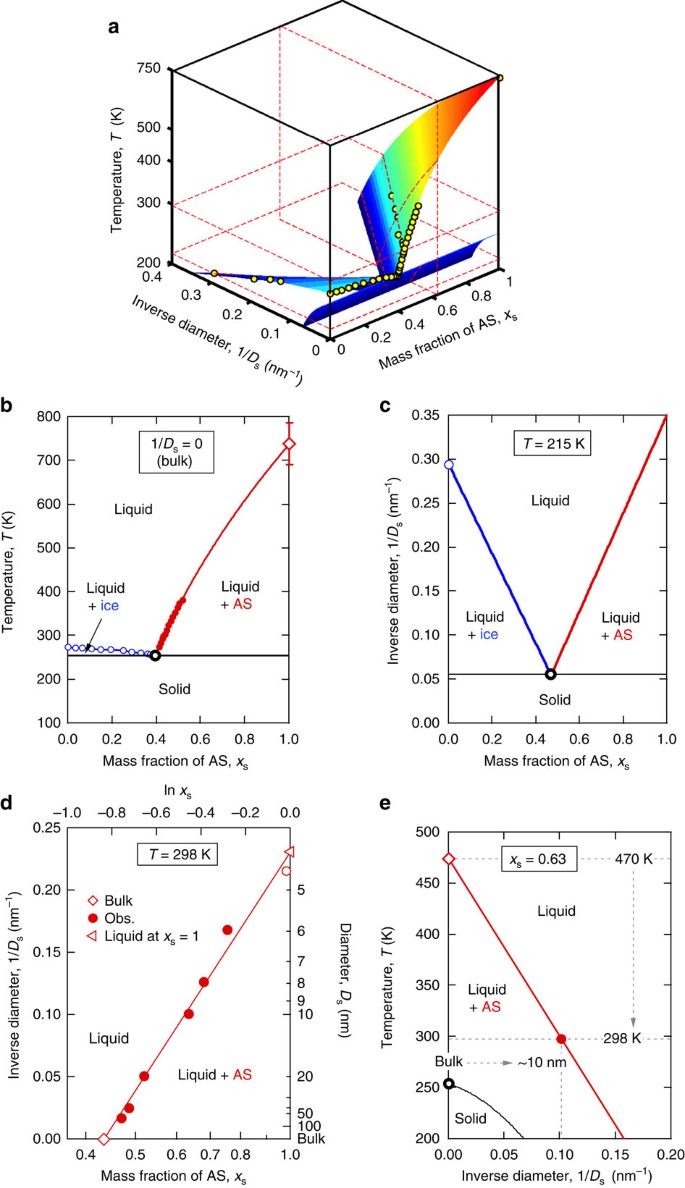
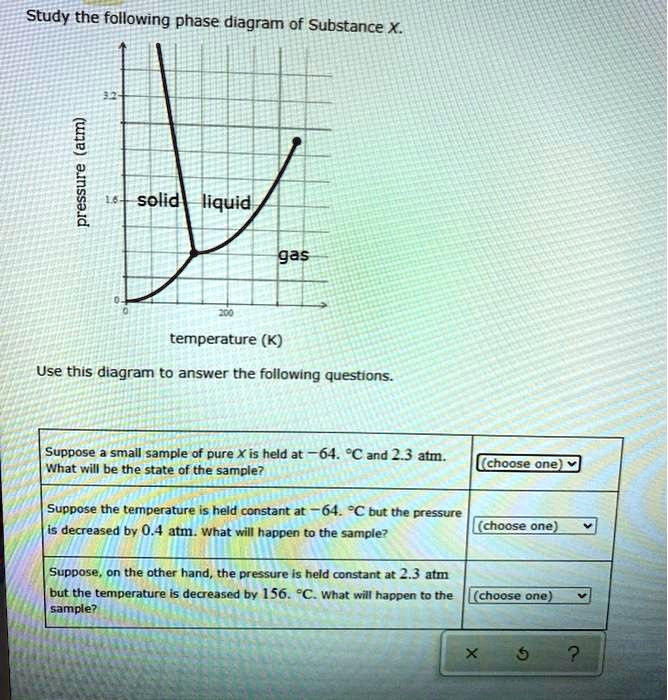
41 Study The Following Phase Diagram Of Substance X.
The phase diagram of a substance can be used to obtain the vapor pressure of that substance at a given temperature. True Below is a phase diagram for compound Q. Consider the phase diagram for substance X shown below. Which of the following is NOT True for substance X? a. At the conditions indicated by point C, substance X will be a liquid. b. At the conditions indicated by point B, substance X will be a gas. c.
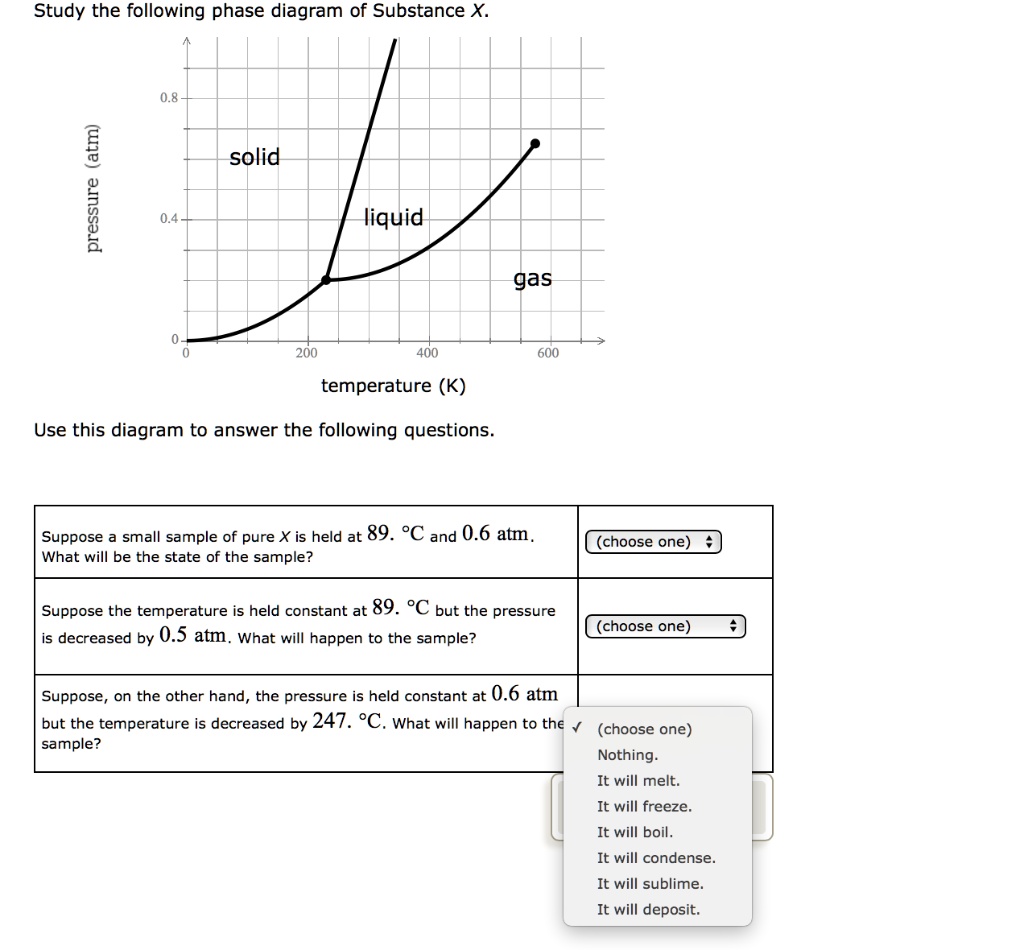
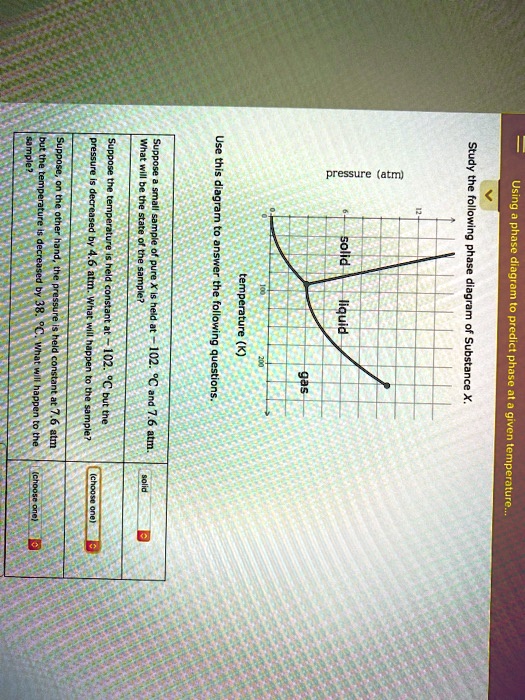
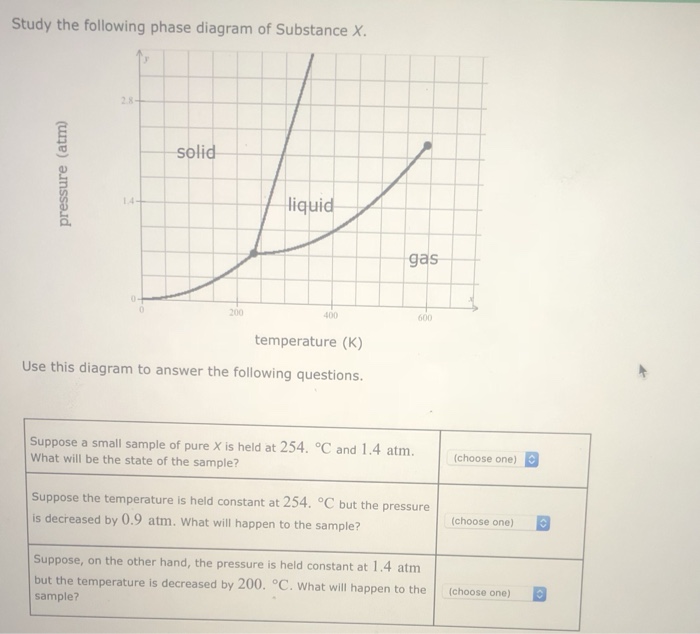
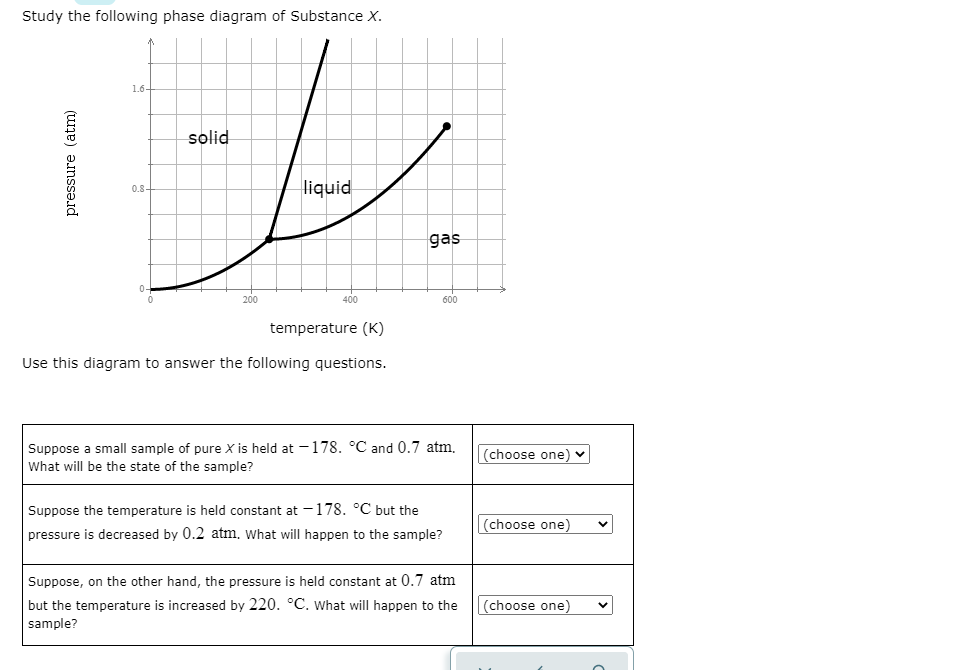
Study the following phase diagram of Substance X. solid pressure (atm) liquid gas temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -202. °C and 1.2 atm. What will be the state of the sample? (choose one) Suppose the temperature is held constant at - 202. °C but the pressure is.

Study the following phase diagram of substance x.
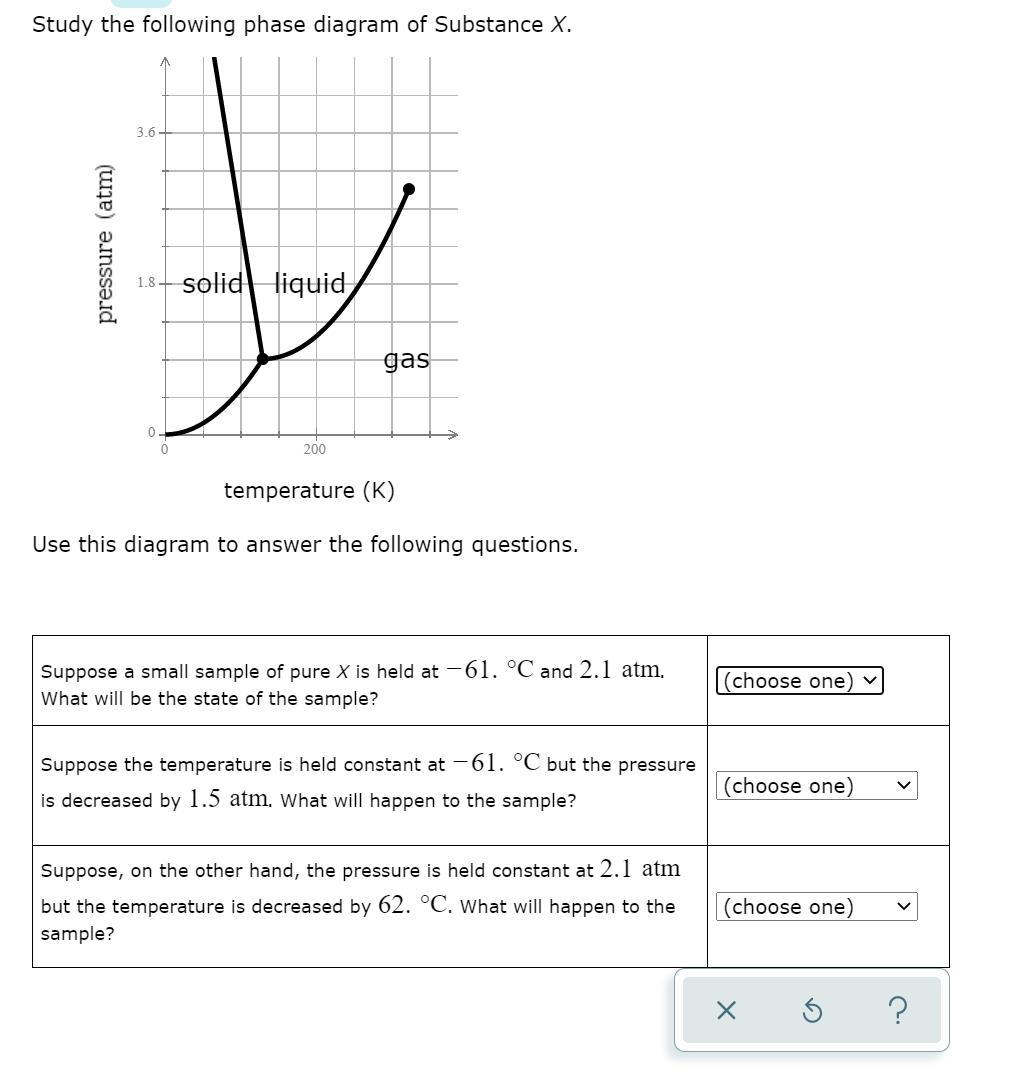
Chemistry Unit 1 Lesson 14. 1. Which curve or point of a phase diagram would indicate the melting point at various temperatures and pressures? 2. In the phase diagram for substance X, what is the triple point of substance X? Refer to the phase diagram for substance X in Problem Set 60: Phase Diagrams, in the Chemistry: Problems and Solutions book. 1.14. Which curve or point of a phase diagram would indicate the melting point at various temperatures and pressures? In the phase diagram for substance X, what is the triple point of substance X? Nice work! You just studied 5 terms! Study the following phase diagram of Substance X. 3.6- 1.8- solid liquid gas 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -61. °C and 2.1 atm, What will be the state of the sample? (choose one) Suppose the temperature is held constant at -61. °C but the pressure (choose.
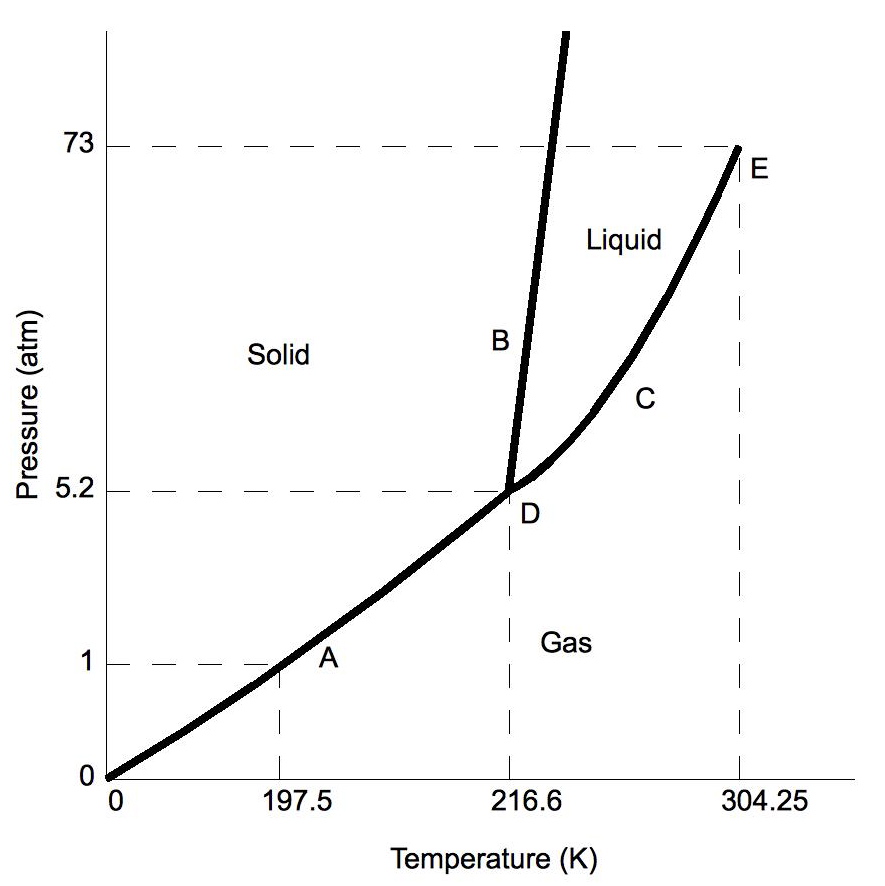
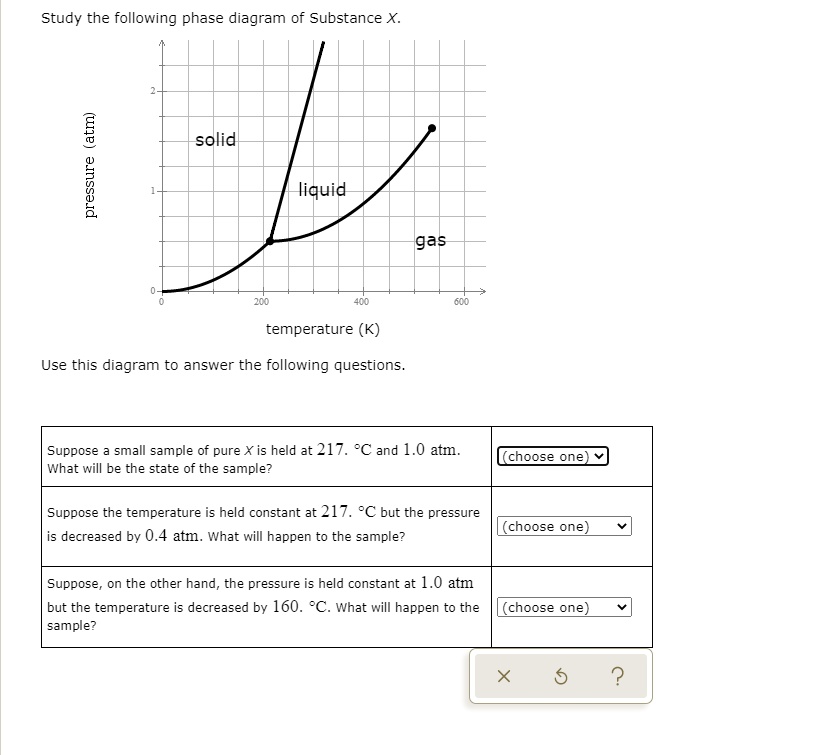
Study the following phase diagram of substance x.. Refer to the phase diagram for substance X in Problem Set 60: Phase Diagrams, in the Chemistry: Problems and Solutions book. In the phase diagram for substance X, what is the triple point of substance X? a.) 0°C, 0 atm b.) 22°C, 4 atm c.) 60°C, 5 atm d.) 29°C, 2.2 atm Based on the phase diagram shown above, which of the following statements are correct? I. Sublimation occurs at a point in the transformation that occurs along a straight line from point A to point F. II. C and E represent points where the gas and liquid phases are in equilibrium. III. ∆Hvap can be measured at point B. IV. Chemistry Unit 1 Lesson 14. 1. Which curve or point of a phase diagram would indicate the melting point at various temperatures and pressures? 2. In the phase diagram for substance X, what is the triple point of substance X? Refer to the phase diagram for substance X in Problem Set 60: Phase Diagrams, in the Chemistry: Problems and Solutions book. Solution for Study the following phase diagram of Substance X. 2.8 solid liquid 14 gas 400 600 temperature (K) Use this diagram to answer the following…
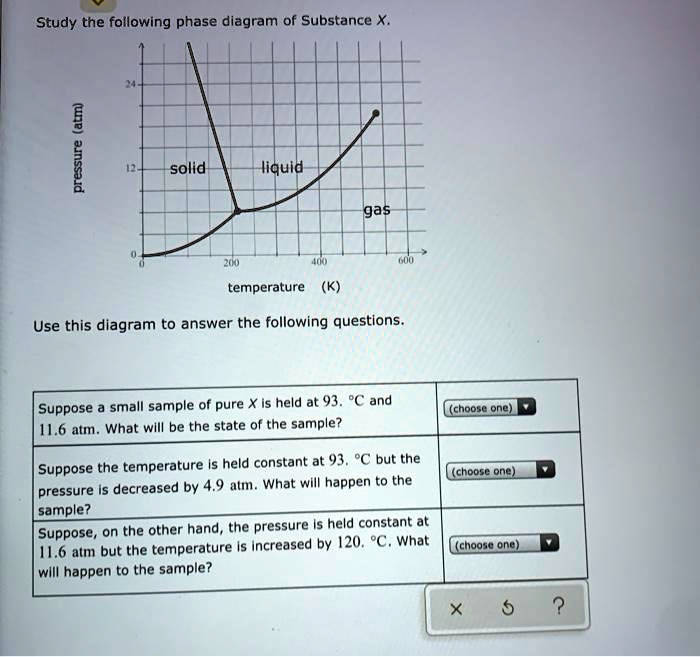
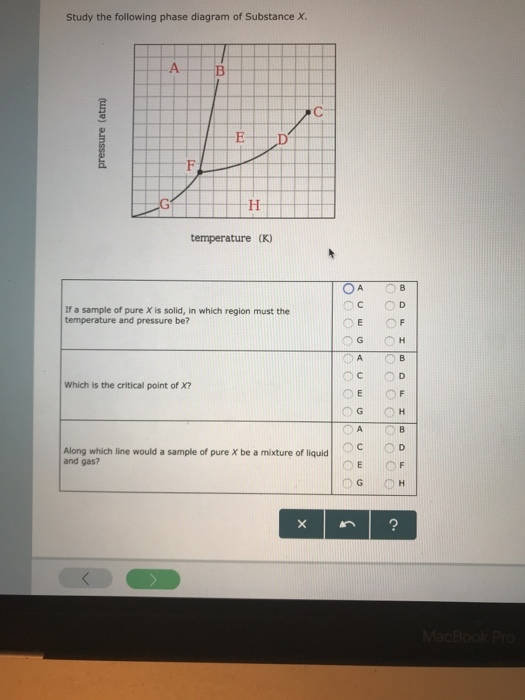
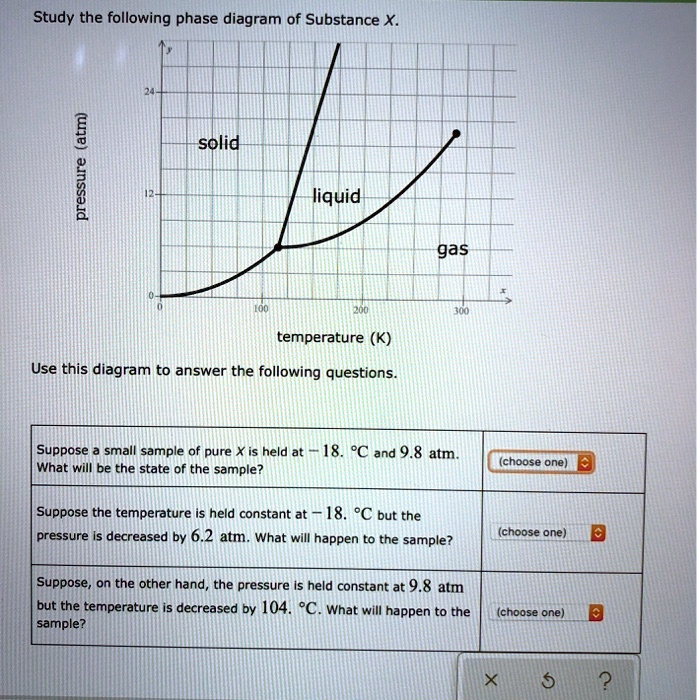
Olo Uw Which line must the temperature and pressure have crossed if a liquid sample of X is observed to freeze? • OF G OH X ? Study the following phase diagram of Substance X. 12 solid pressure (atmi liquid gas 100 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -84. "C and 7.1 atm. Phase Diagrams. Open the phase diagram for CO2 given in the introduction again. Use the phase diagram for CO2 in the interactive activity and determine which of the following statements are correct. CO2 is a gas under normal conditions of temperature and pressure. All three phases of CO2 exist simultaneously at the triple point. Transcribed image text: Study the following phase diagram of Substance X pressure (atm) solid liquid gas temperature (K) Use this diagram to answer the following questions, Suppose a small sample of pure X is held at 71. C and 19.2 atm. what will be the state of the sample? (choose one Suppose the temperature is held constant at 71. "C but the pressure is decreased by 15 2 atm. Phase Diagram Worksheet Name: A phase diagram is a graphical way to depict the effects of pressure and temperature on the phase of a substance: The CURVES indicate the conditions of temperature and pressure under which "equilibrium" between different phases of a substance can exist. BOTH phases exist on these lines:
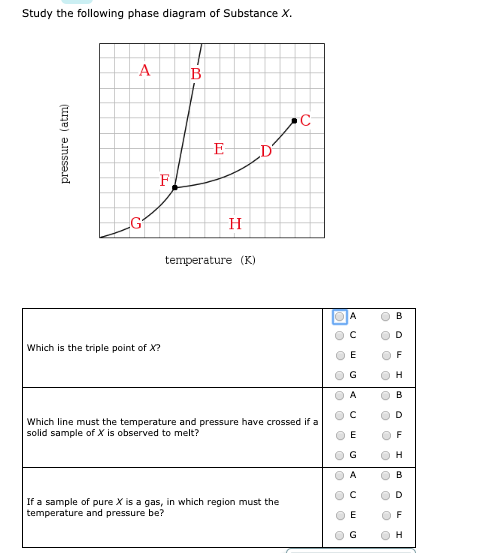
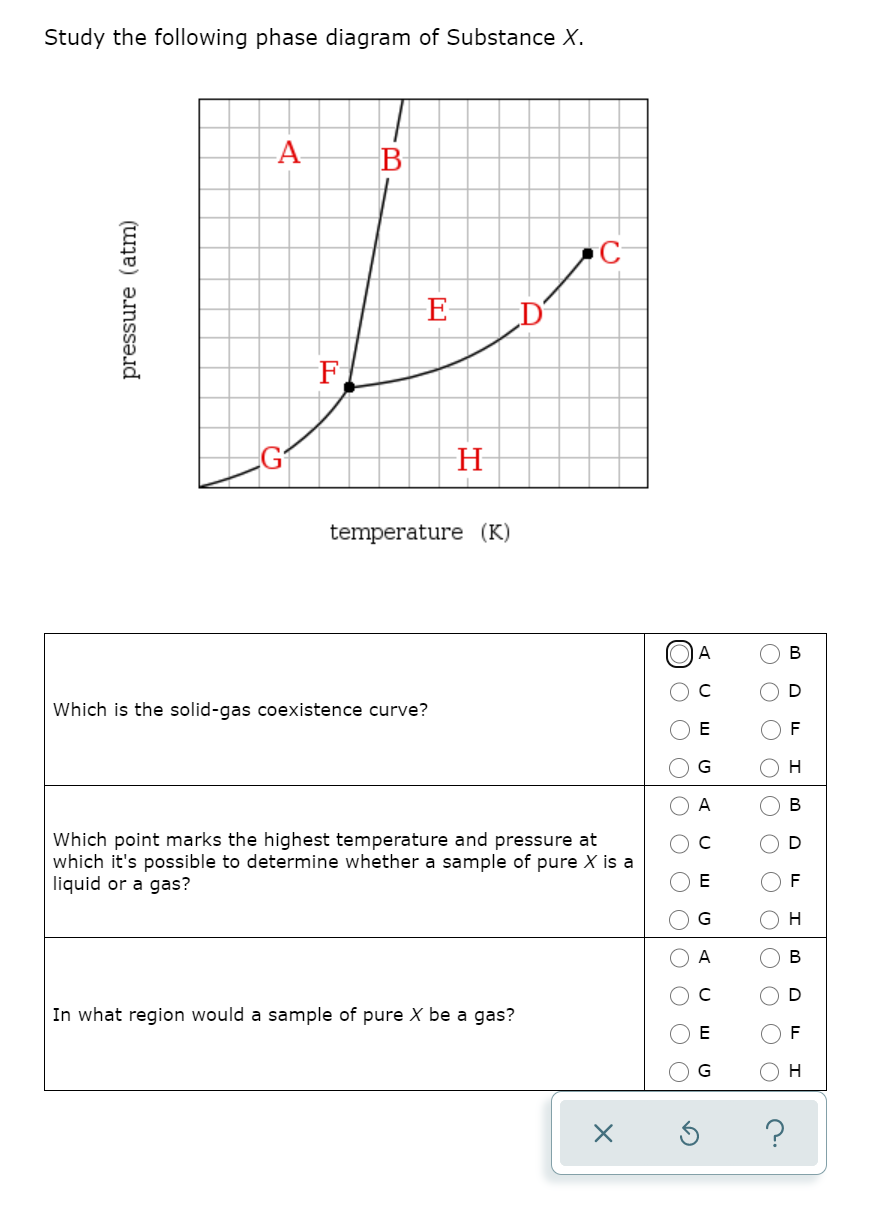
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures. Answer to Study the following phase diagram of Substance X. AB. Transcribed image text: Study the following phase diagram of Substance X. AB pressure (atm) E D temperature (K) Which region includes the highest pressures and lowest temperatures at which the phase of X is known? OH At which point would a sample of pure X be a mixture of solid, quid and gas? Transcribed image text: Study the following phase diagram of Substance X. solid liquid gas 100 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at-79. °C and 1.4 atm. What will be the state of the sample? (choose one) Suppose the temperature is held constant at-79. °C but the pressure is decreased by 0.4 atm. Science. Chemistry. Chemistry questions and answers. Study the following phase diagram for substance X. If a sample of pure X is a gas, in which region must the temperature and pressure be? A B pressure (atm) E H Tetapetatur. Question: Study the following phase diagram for substance X. If a sample of pure X is a gas, in which region must the.
A phase diagram is a graph which shows under what conditions of temperature and pressure distinct phases of matter occur. The simplest phase diagrams are of pure substances. These diagrams plot pressure on the y-axis and temperature on the x-axis. Although phases are conceptually simple, they are difficult to define precisely.
Study the following phase diagram of Substance X. 3.6- 1.8- solid liquid gas 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -61. °C and 2.1 atm, What will be the state of the sample? (choose one) Suppose the temperature is held constant at -61. °C but the pressure (choose.
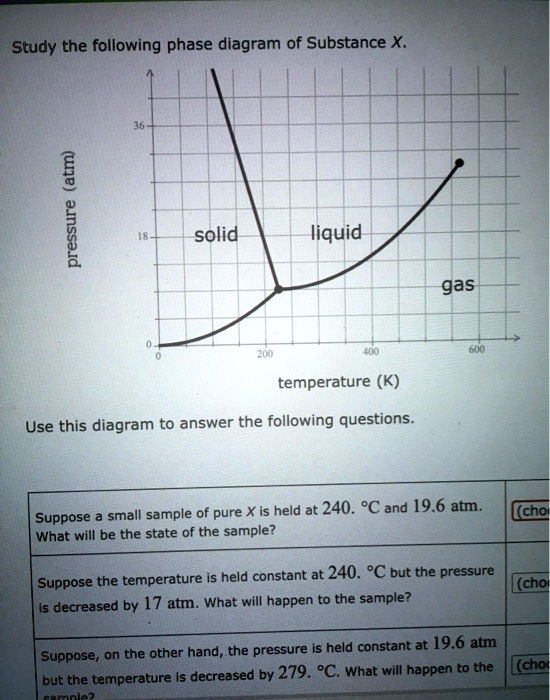
Solution for Study the following phase diagram of Substance X. 1.6+ solid 0.8- liquid gas 200 400 600 temperature (K) Use this diagram to answer the following…
Below is a phase diagram for a substance. Study the following phase diagram of substance x. Phase diagrams wiva k12 chemistry. Which curve or point of a phase diagram would indicate the melting point at various temperatures and pressures. Problems and solutions book. Dont have ref 29c 22 atm 3. This is the phase diagram for a typical pure.
Transcribed image text: Study the following phase diagram of Substance X. 2.4 solid 121 liquid gas 200 400 600 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at 109. °C and 1.5 atm. What will be the state of the sample? liquid Suppose the temperature is held constant at 109. °C but the pressure is decreased by 1.1 atm.
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid.
Study the following phase diagram of Substance X. solid liquid gas pressure (atm) check_circle.
Transcribed image text: 3. Study the following phase diagram of Substance X. AB temperature (K) In what region would a sample of pure X be liquid? If a sample of purex is observed to be a mixture of solid, liquid l and gas, which point marks the temperature and pressure? o c o D Which is the solid-liquid coexistence curve?
The temperature at which the components are completely miscible is given by following the isopleth upwards and noting the temperature it enters the one-phase region of the diagram. Answer: We denote hexane by H and nitrobenzene by N. The point x N = 0.41, T = 290, occurs in the two-phase region of the diagram.
1.14. Which curve or point of a phase diagram would indicate the melting point at various temperatures and pressures? In the phase diagram for substance X, what is the triple point of substance X? Nice work! You just studied 5 terms!
Phase Diagram: Phase diagram shows the thermodynamic conditions at which various states of the substance i.e solid/liquid and gaseous states coexist at equilibrium.
Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.
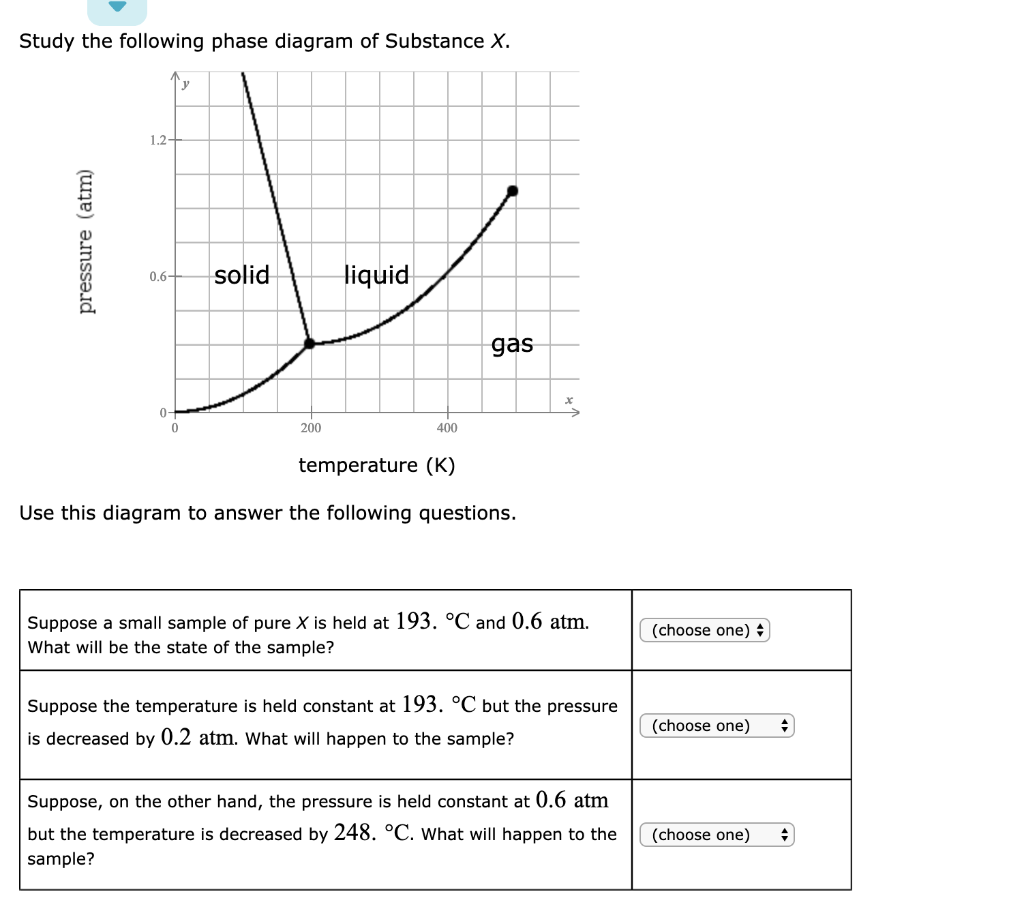
Study the following phase diagram of Substance X. 1.6- pressure (atm) 0.8 solid liquid gas 200 400 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -48. °C and 0.9 atm. What will be the state of the sample?
Temperature k study the following phase diagram of substance x. Phase diagrams wiva k12 chemistry. This is the phase diagram for a typical pure substance. Which point marks the highest temperature and pressure at which its possible to determine whether a sample of pure x is a liquid or a gas. Phase diagram is a graphical representation of the.
Study the following phase diagram of Substance X. 1.2 solid pressure (atm) 0.0 liquid gas 100 200 300 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at - 121. °C and 0.5 atm What will be the state of the sample?















:max_bytes(150000):strip_icc()/phasediagram-56a129b35f9b58b7d0bca3ea.jpg)
0 Response to "41 Study The Following Phase Diagram Of Substance X."
Post a Comment