40 Lewis Electron Dot Diagram Definition
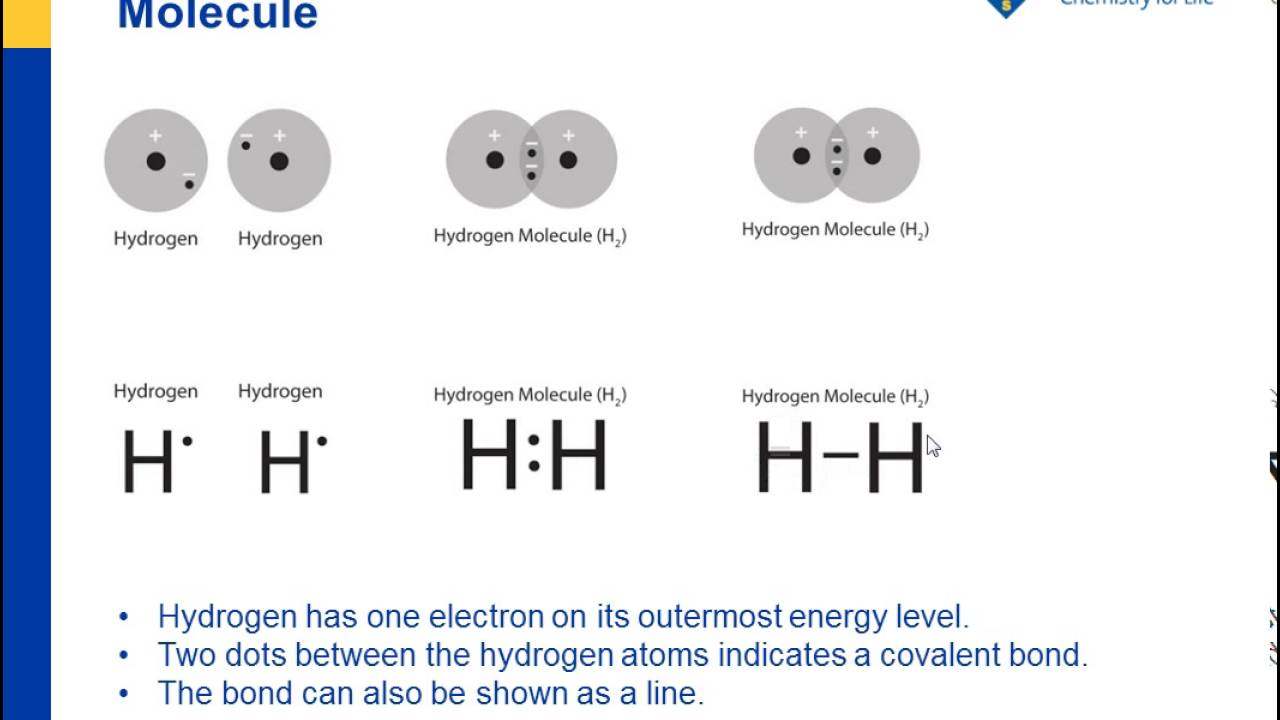
Lewis structure definition. A Lewis structure is a structural representation of a molecule where dots are used to show electron. positions around the atoms and lines or dot pairs represent covalent bonds between atoms. They are also known as are also called Lewis dot diagrams, electron dot diagrams, Lewis dot formulas, or electron dot formulas. Lewis structures go by many names, including Lewis electron dot structures, Lewis dot diagrams, and electron dot structures. All these names refer to the same sort of diagram, which is intended to show the locations of bonds and electron pairs.
A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps to make a prediction about the geometry of a molecule.
Lewis electron dot diagram definition
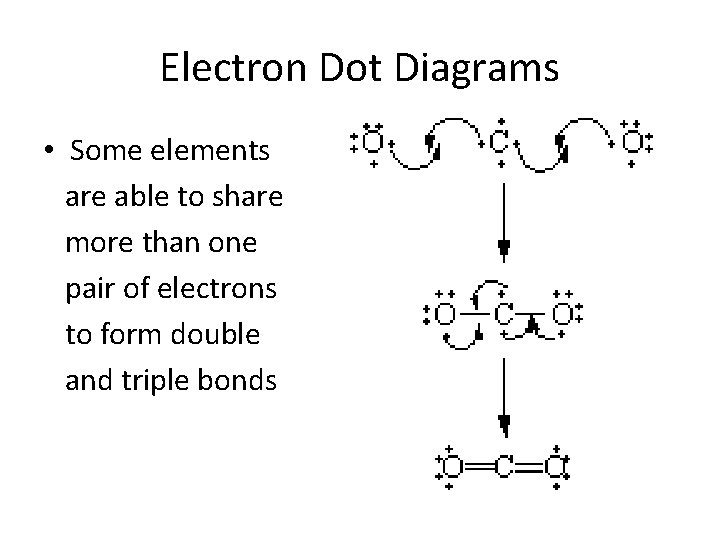
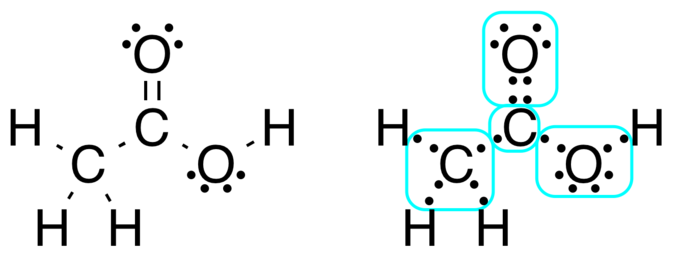
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
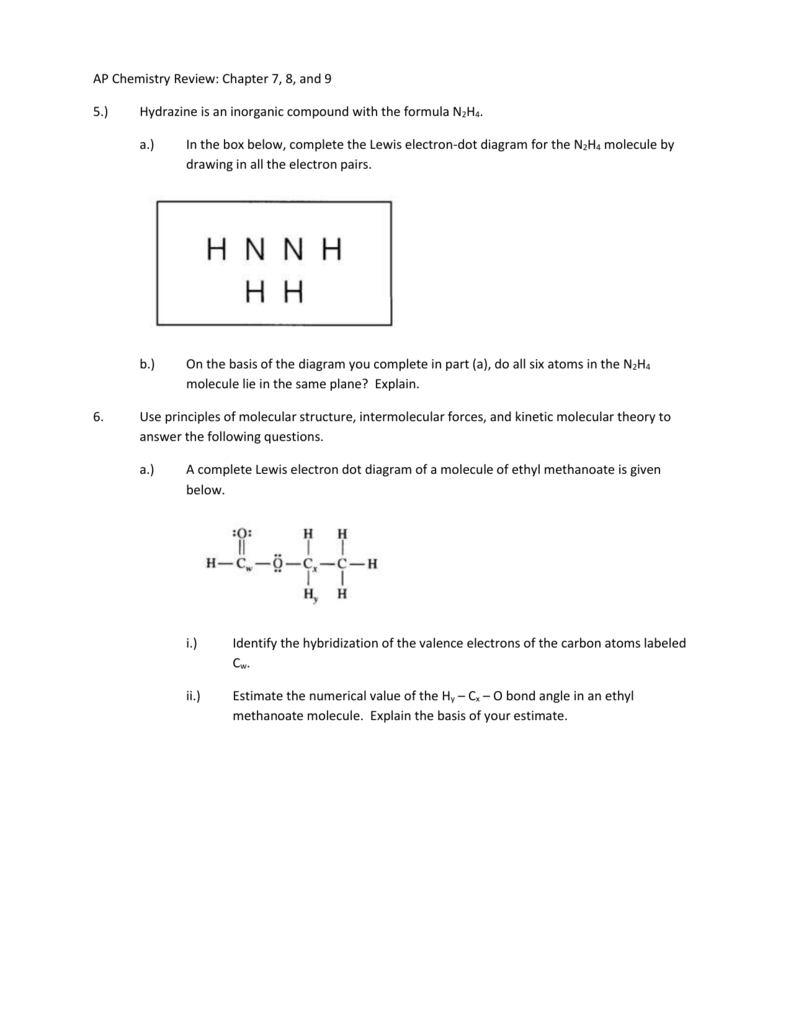
Lewis electron dot diagram definition. Lewis Dot Structure Definition. Lewis dot structure definition: a visual way to clearly depict the connection of atoms and the electrons present in a molecule.With a carbon Lewis dot structure, one can see how the atoms in a molecule are bonded together, which gives us more information about the structure than the molecular formula. Lewis structure. [ ′lü·is ‚strək·chər] (chemistry) A structural formula in which electrons are represented by dots; two dots between atoms represent a covalent bond. Also known as electron-dot formula; Lewis formula. Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. Lewis structure in chemistry. Lewis structures, also called electron-dot structures or electron-dot diagrams, are diagrams that show the bonding between atoms of a molecule, and the lone pairs of.
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons. Electron Dot Structure: Electron dot structure is simply Lewis structure which shows bonding present between the atoms of molecule. It also shows lone pairs of electrons if present in a molecule. Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.
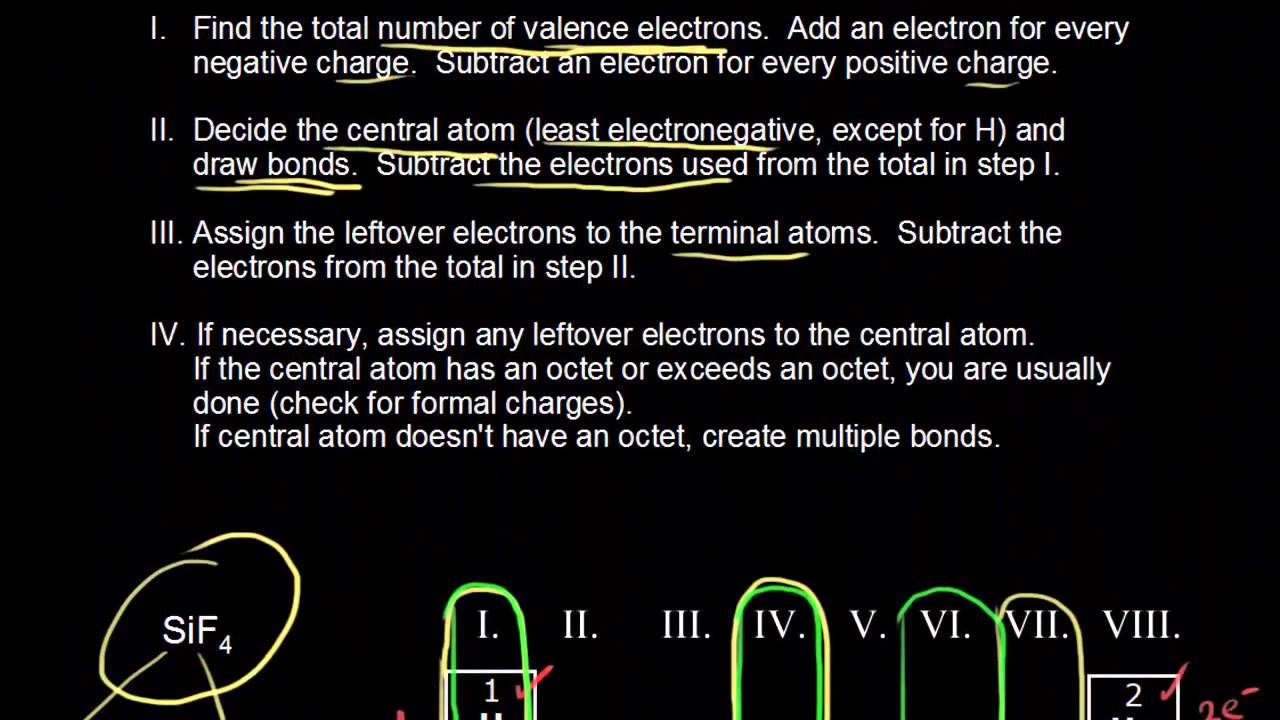
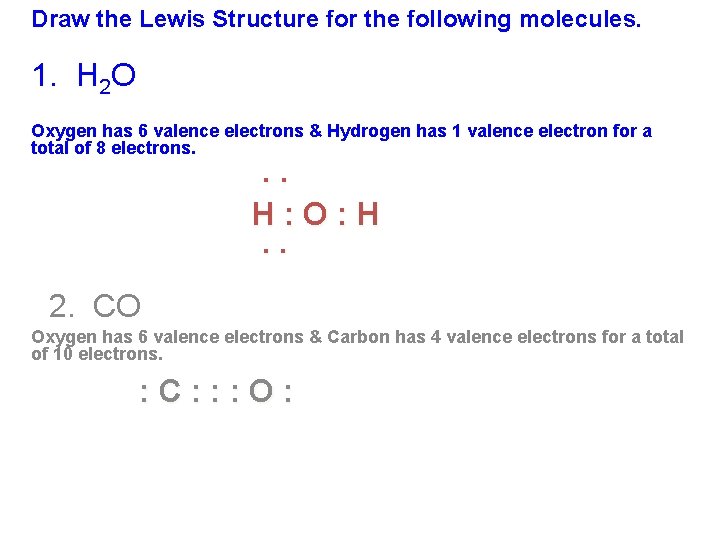
Draw the Lewis dot structure for CO. The number of valence electrons is 4 + 6 = 10 electrons or 5 pairs. Since both C and O allow multiple bonds we can still follow the octet and write: If there is not enough electrons to follow the octet rule, then the least electronegative atom is left short of electrons. Draw the Lewis dot structure for BeF2. The Lewis dot structure was named after the great American chemist Gilbert Newton Lewis.If the Molecular formula of a compound is known, one can draw its electron dot structure or lewis dot structure and can define the nature and position of its bond and molecules respectively. Here we will discuss more about the concept with some solved examples and questions. Definition of Lewis formula (electron dot or Lewis structure) Molecular structure in which the valency electrons are shown as dots so placed between the bonded atoms that one pair of dots represents two electrons or one (single) covalent bond , e.g. A double bond is represented by two pairs of dots, etc. Dots representing non-bonded outer-shell. The Lewis Electron-Dot Symbols of Elements. Gilbert N Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen the Bohr's diagram for the first 18 elements. Sometimes it is more convenient to represent the elements by its Lewis electron dot symbol.
Electron Dot Diagram Definition Resonance Chemistry Socratic. Electron Dot Diagram Definition Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry. Electron Dot Diagram Definition H2s Lewis Dot Diagram Wiring Diagram Directory. Electron Dot Diagram Definition The Lewis Dot Structure For… Continue Reading →
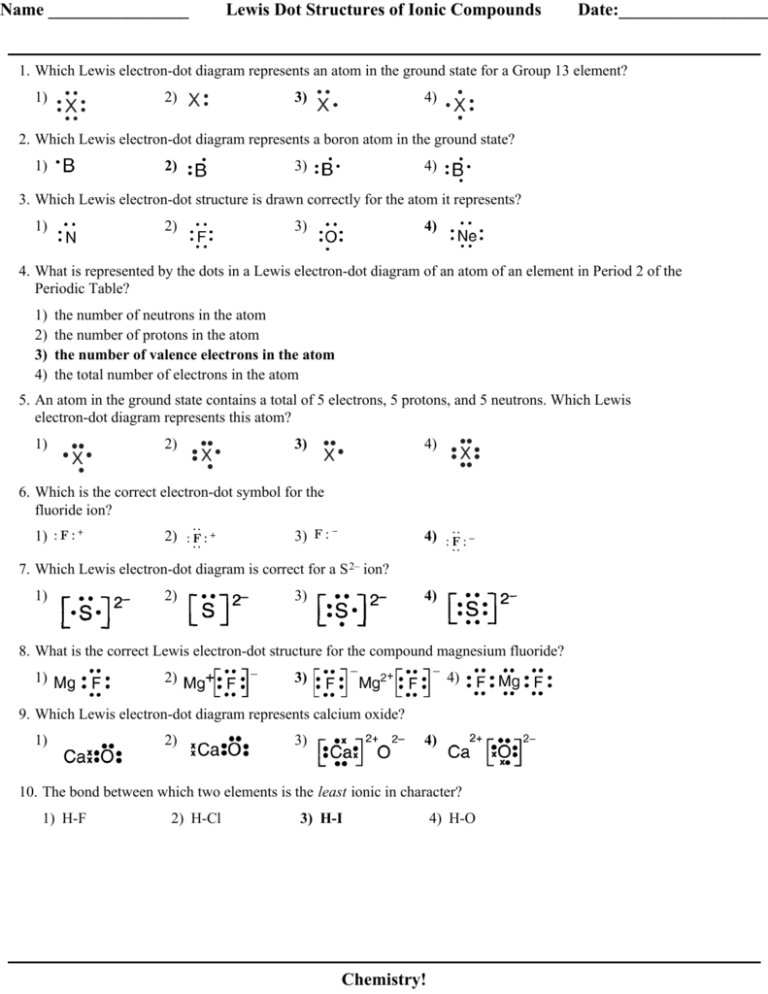
Q. This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2.
Molecular structure in which the valency electrons are shown as dots so placed between the bonded atoms that one pair of dots represents two electrons or one covalent (single) bond, e.g. L03513.png A double bond is represented by two pairs of dots, etc. Dots representing non-bonded outer-shell electrons are placed adjacent to the atoms with.
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 –3 b. NO 3 – d. CO 3 2– 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Lewis Structures: As valence electrons are significant to an atom's reactivity, it is essential to represent it by simple diagrams. Lewis structures, here, comes into the picture where the valence electrons present in an atom are represented as dots. Hence, these structures are also known as electron dot diagrams.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.
Finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Those are lewis dot structures. Let's learn how...
Lewis Dot Structures - Definition and Example. Lewis structure is basically a graphic representation of the electron distribution around an atom. The major reason why learning Lewis dot structure is important is that it helps in predicting the number and type of bonds which can be formed around an atom. It also helps in predicting the geometry.
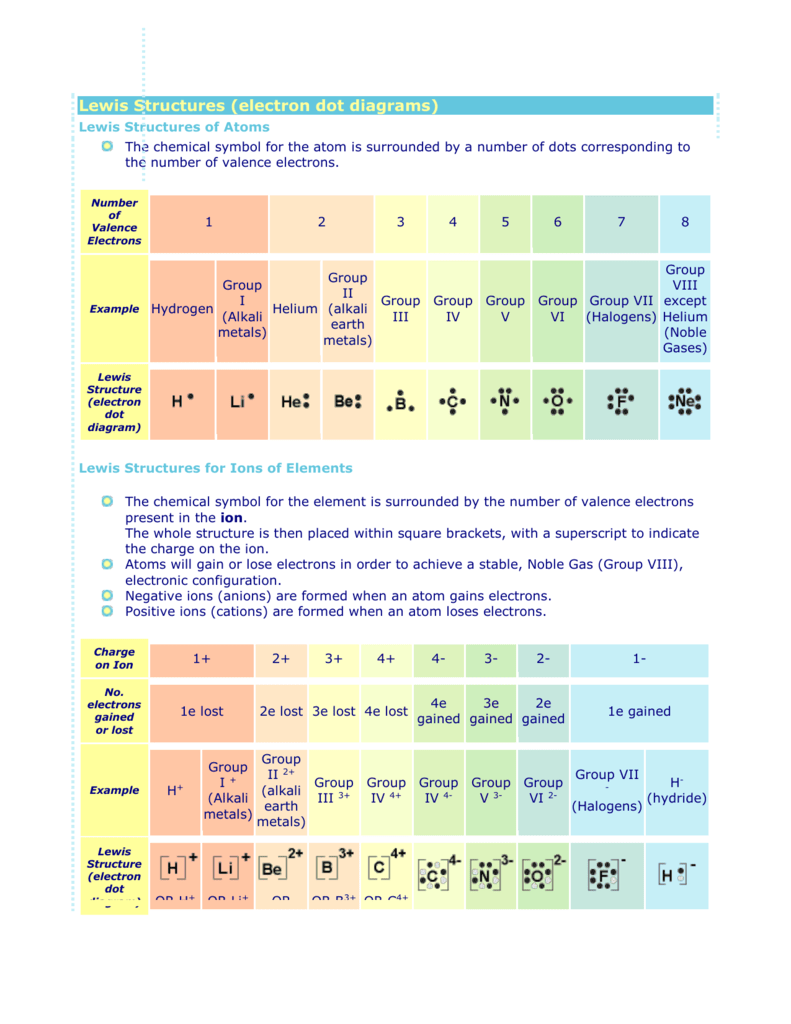
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are.
The valence electron for any atom can be counted using the Lewis electron dot diagram. The Lewis dot structure represents the valence electrons of atoms. The Lewis structure for an atom is drawn by placing a dot around the atom for each valence electron that is present.
Nitrate ion lewis dot structure. In brief we need to master 4 steps for making a correct Lewis dot structure. Count total valence electrons in the molecule or ion. Select the central atom and make a skeleton of the molecule or ion. Complete the octet of the most electronegative atom with minimum formal charges.
To use Lewis electron dot symbols to predict the number of bonds an element will form. At the beginning of the 20th century, the American chemist G. N. Lewis (1875-1946) devised a system of symbols—now called Lewis electron dot symbols. A system that can be used to predict the number of bonds formed by most elements in their compounds.
In a Lewis Structure, a shared pair of electrons is depicted with a _____, an unpaired electron is depicted with a _____ by itself, and a lone pair of electrons is depicted with a _____ next to each other.
Definition of Lewis structure (electron dot structure; dot structure) Search Web Search Dictionary Get Babylon's Dictionary & Translation Software Free Download Now!
And thus Lewis dot treatment distributes the valence electrons. And we can easily find the number of valence electrons for a given atom by noting its Group number in the Periodic Table, which number gives required the number of electrons. For a simple example, consider ammonia, N H 3, the which has 5 nitrogen valence electrons, and 3 electrons.
Lewis structure. [ ′lü·is ‚strək·chər] (chemistry) A structural formula in which electrons are represented by dots; two dots between atoms represent a covalent bond. Also known as electron-dot formula; Lewis formula.



/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)







/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)












0 Response to "40 Lewis Electron Dot Diagram Definition"
Post a Comment