40 Lewis Dot Diagram For Nh3
Step-3: Lewis dot Structure for NH3 generated from step-1 and step-2. Connect the exterior and core central atom of the NH3 molecule with three single bonds (N-H). In this stage, use three hydrogen atoms on the outside of the NH3 molecule to the central nitrogen atom in the middle. August 14, 2018 - Something went wrong. Wait a moment and try again
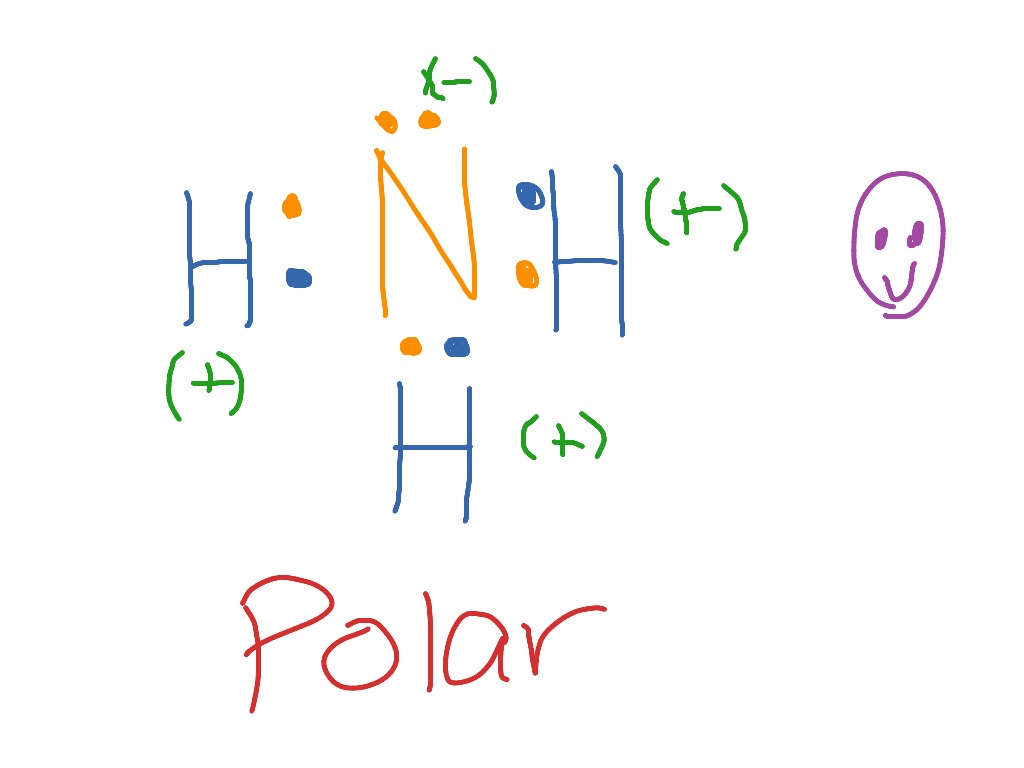
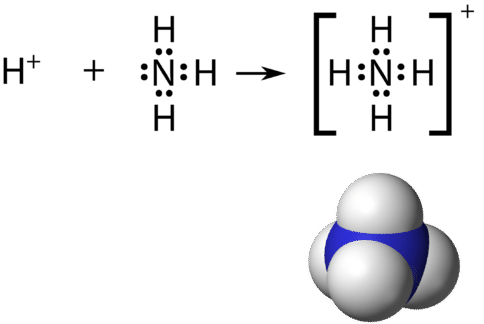
May 5, 2018 - Have a look here... The Lewis structure of ammonia, NH_3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.

Lewis dot diagram for nh3
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation Which of the following is true regarding the Lewis diagram for ammoni a? (2 points) a. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has no lone pairs of electrons. b. Question: Draw the Lewis electron dot diagram for ammonia (NH3) on a sheet of scrap paper. You may consult the periodic table on Zoom to. Drawing the Lewis Structure for NH 3 ( Ammmonia) Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.
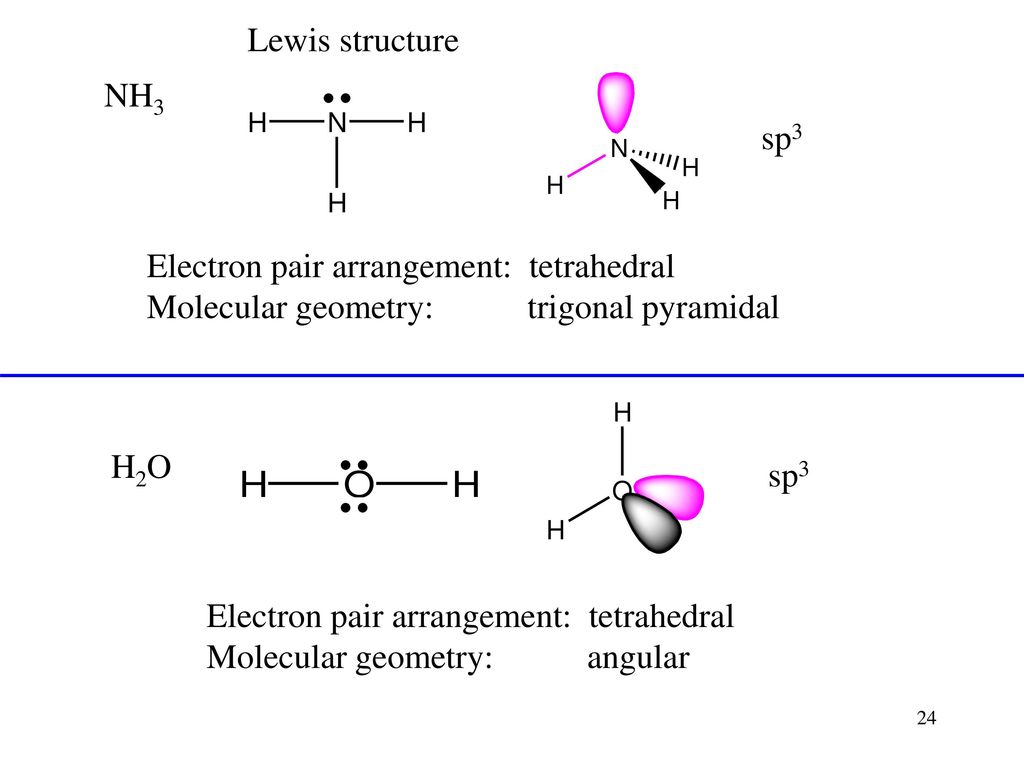
Lewis dot diagram for nh3. What is the Lewis dot structure for ammonia? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of […] In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation In the Lewis dot structure for NH3, the central atom of the molecule has three atoms bonded to it and one lone pair of electrons. The shape of an NH3 molecule is bent. tetrahedral. trigonal planar. trigonal pyramidal. I quickly take you through how to draw the Lewis Structure of Ammonia, NH3. I also go over hybridization and bond angle.
Lewis Dot Diagram Of Nh3. Ammonia has the formula NH3. It's highly soluble in water because it's a polar substance. The Lewis structure for a molecule represents the total valence. The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Lewis Structures for NH3. Apr 3, 2015 - A step-by-step explanation of how to write the Lewis Dot Structure for NH4+ (Ammonium Ion).For the NH4+ Lewis structure, calculate the total number of valenc... Step 4: There were 8 valence electrons in step 1 and 8 electrons in the Lewis dot structure. Application: Use the atom cards and Cheerios to build the molecule before drawing it. 1. 2. 3. Complete the Lewis dot Structure for CC14 Complete the Lewis dot structure for NH3 Complete the Lewis dot structure for H20 -c-CI: The electron-dot structure (Lewis structure) for which of the following molecules would have two unshared pairs of electrons on the central atom. H2S.. NH3, and H2O is the best account for by the. Increasing the number of unshared pairs of electrons. the smaller ions have a stronger coulombic attraction to water.
Alternate lewis dot structure of water. 10+ Nh3 Lewis Structure. This is the reason why ammonia acts as a lewis base, as it can donate those electrons. (1) you have two chlorine atoms. Bonding and structure in covalent compounds. Electron dot structures or lewis dot formula can be drawn if the molecular formula of the compound is known. Drawing the Lewis Structure for NH 3 ( Ammmonia) Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. Similarly, it is asked, what is the Lewis dot structure for nh3? Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. Hey everyone, welcome to the Mentor Center! In today's video, I draw out the Lewis dot structure for NH3, commonly known as ammonia.👍 Like 📽️ Subscribe.
Lewis Dot of Ammonia · Back70 More Lewis Dot StructuresSince all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule
ShowMe is an open learning community featuring interactive lessons on a variety of topics.
The Lewis dot structure are the diagrams that represent the valence electrons of atoms in a molecule. It is used to show how the electrons are arranged around individual atoms inside a molecule. In this structure, as the name suggests, electrons are shown as dots and a line for bonding electrons between the two atoms.
January 1, 2019 - Learn what the Lewis Dot Structure for NH3 is in this post by makethebrainhappy.
Answer (1 of 3): One thing you know right off the bat is that nitrogen is in the middle. Hydrogen only has one valence electron, so it will just have the one bond. Draw N in the middle and draw the "H"s around it, one on each side and one either on the top or the bottom (it doesn't matter which)....
Lewis dot diagram for nh3. In the nh 3 lewis structure and all structures hydrogen goes on the outside. The lewis structure for nh3 is one of the most common lewis structures to show up on general chemistry tests. For the nh3 lewis structure calculate the total number of valence electrons for the. Drawing the lewis structure for nh 3.
August 1, 2016 - Answer (1 of 3): One thing you know right off the bat is that nitrogen is in the middle. Hydrogen only has one valence electron, so it will just have the one bond. Draw N in the middle and draw the “H”s around it, one on each side and one either on the top or the bottom (it doesn’t matter.
Video Bokep Indo Terkini - Nonton Dan Unduh Video Bokep Indo Lewis dot diagram for nh3. Video Bokep ini yaitu Video Bokep yang terkini di November 2021 secara online Film Bokep Igo Sex Abg Online , streaming online video bokep XXX Cuma-cuma , Nonton Film bokep jilbab ABG Perawan
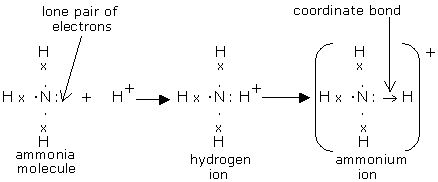
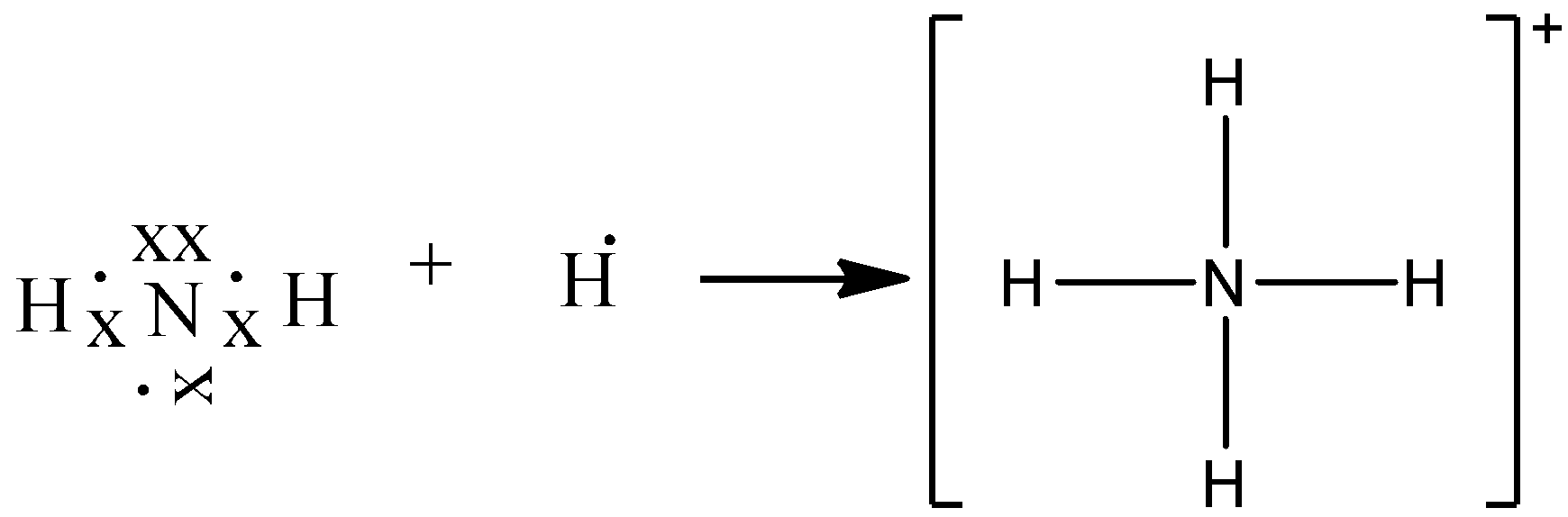
The Lewis Dot Structure for NH4+ (Ammonium) is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence shell, with nitrogen having access to eight electrons and each hydrogen having access to two (this is why hydrogen only needs two).
Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
A stepbystep explanation of how to write the lewis dot structure for nh3 (ammonia or nitrogen trihydride). For (nh4)2so4 we have an ionic compound and need to take that into account when draw the lewis structure. A simple method for drawing the lewis structure ammonia. We'll first ammonium ion (nh4+).
Which of the following is true regarding the Lewis diagram for ammoni a? (2 points) a. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has no lone pairs of electrons. b. Question: Draw the Lewis electron dot diagram for ammonia (NH3) on a sheet of scrap paper. You may consult the periodic table on Zoom to.
FG20 15 01UN JPG. lewis structure for nh3 ammonia university maryland lewis structures for nh3 step by step tutorial for drawing the lewis structure for ammonia. Structure Validation. lewis dot of ammonia nh3 ap chemistry 70 more lewis dot structures since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule the.
Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Steps of drawing the lewis structure of NH3 is explained in detail in this tutorial.
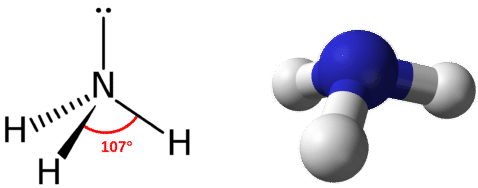
The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3. Secondly, what is the shape of nh3.
In the box below, draw the electron-dot (Lewis) structure of carbon di0>äde. In the box below, draw a Lewis electron-dot diagram for a molecule of phosphorus trichloride, PC13 (c) ammonia 60) 61) 62) 65) In the box provided, draw a Lewis electron-dot diagram for a molecule of chlorine, Ch. in terms of electrons, why the bonding in NaCl is Ionic.
Lewis Dot of the Ammonium Ion · Back70 More Lewis Dot StructuresSince all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape. Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton.
A video explanation of how to draw the Lewis Dot Structure for Ammonia, along with information about the compound including Formal Charges, Polarity, Hybrid.
Spell. Test. PLAY. Match. Gravity. (A) H2O (B) NH3 (C) BH3 (D) CH4 (E) SiH4. Has a central atom with less than an octet of electrons. Click card to see definition 👆. Tap card to see definition 👆.
The Lewis structure for NH3 is.The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom. The N atom then has two dots on the unconnected side. NH3, commonly known as ammonia, is arranged as a T-shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities.
What kind of Dot and cross diagram is NH3? Draw the dot and cross diagram for ammonia, NH3. Nitrogen has five outer electrons because it is in group 5. Draw the dot and cross diagram for methane, CH4. Carbon has four outer electrons. Some molecules contain a double bond, which consists of two shared pairs of electrons.
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale...
NH3 Lewis Structure, Geometry, and Hybridization. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.
Transcript: OK, this is Dr. B. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15 so it has 5 valence electrons, and then Hydrogen is in group 1. It has one valence electron, but we have 3 Hydrogens, so let's mutiply.
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
September 17, 2020 - The diagram of the NH3 Lewis structure is drawn using crosses and dots around the picture of an atom, by and large in pairs. Additionally, the lines show bond formation between the particles where it shows if a single, double bond, or triple bond has been formed.
Electron Dot Structure of NH3 by Jeff Bradbury - February 17, 2012 - Lewis Electron Dot Structure for ammonia molecule NH3







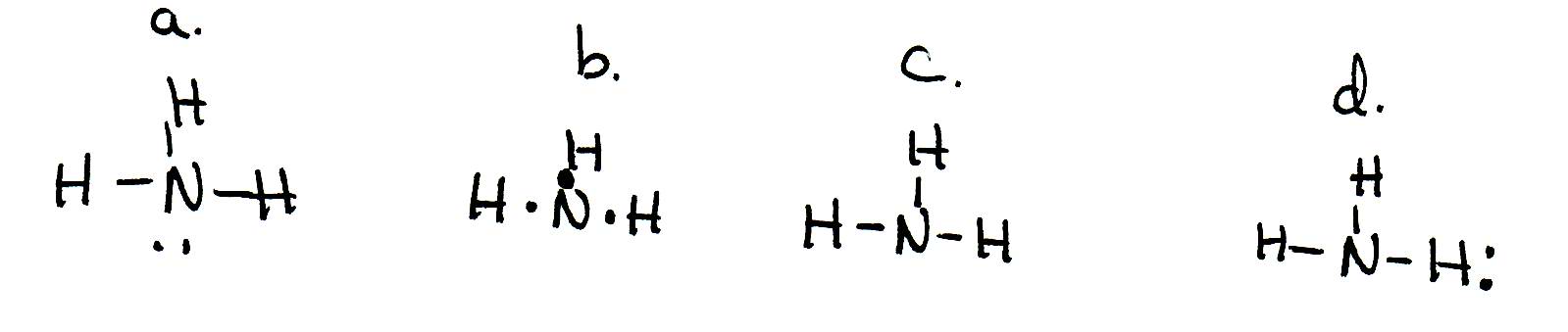



















0 Response to "40 Lewis Dot Diagram For Nh3"
Post a Comment