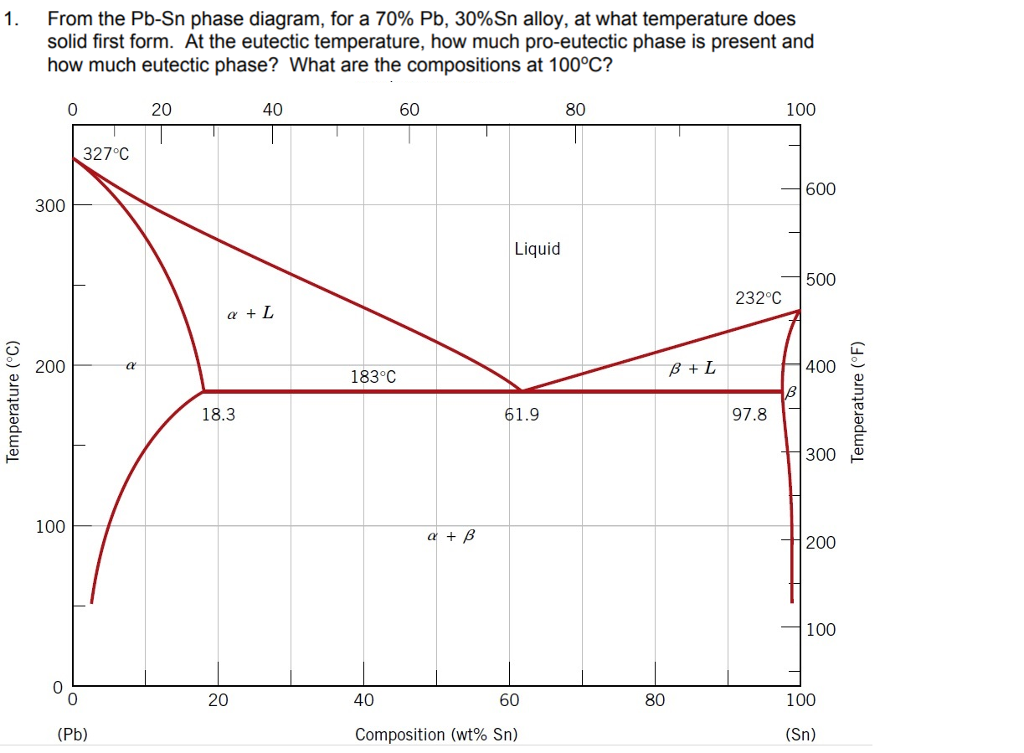
43 Pb-sn Phase Diagram
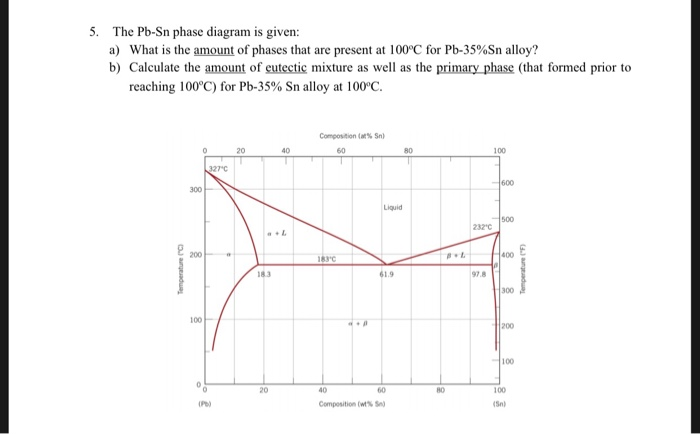
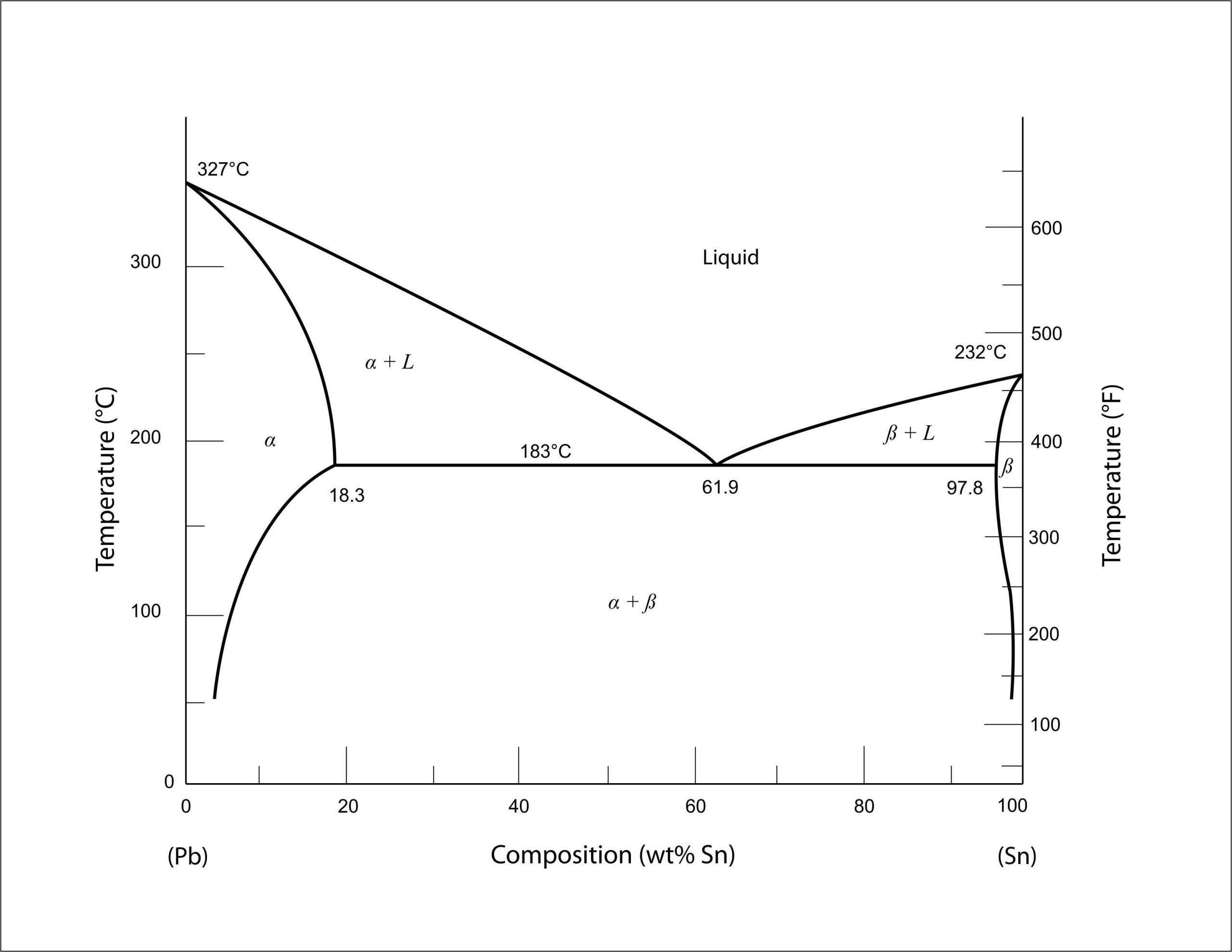
Alloy Phase Diagrams 9 (1988) 144-152. H. Ohtani, K. Okuda and K. Ishida, J. Phase Equilibria 16 (1995) 416-429. Calculated Invariant Equilibria. Reaction. Phase. Mass % Pb. Mass % Sn. L -> (Pb) + (Sn) 182.2 o C. In the Pb-Sn phase diagram on the right, an alloy contains 64 wt% proeutectic α and 36 wt% of eutectic α+β at 180oC -ΔT, find the average composition of this alloy A. 34.6% Sn B. 65.4 % Sn C. 64 % Sn D. 27.3% Sn Answer: Since the alloy contains proeutectic , so the average composition of this alloy must vary between 19.2%( ) and.
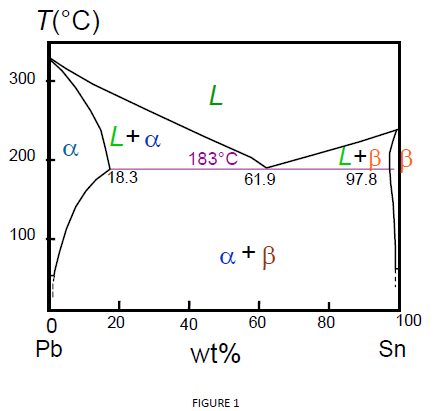
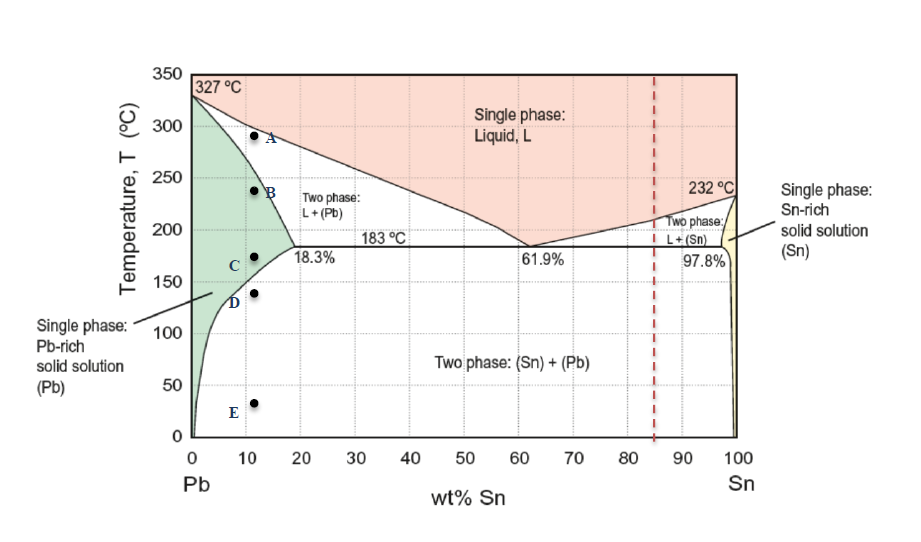
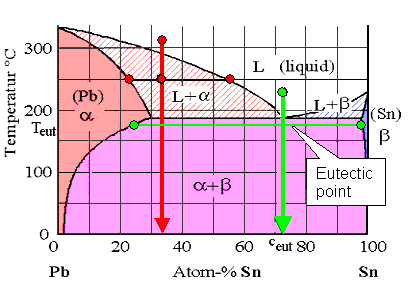
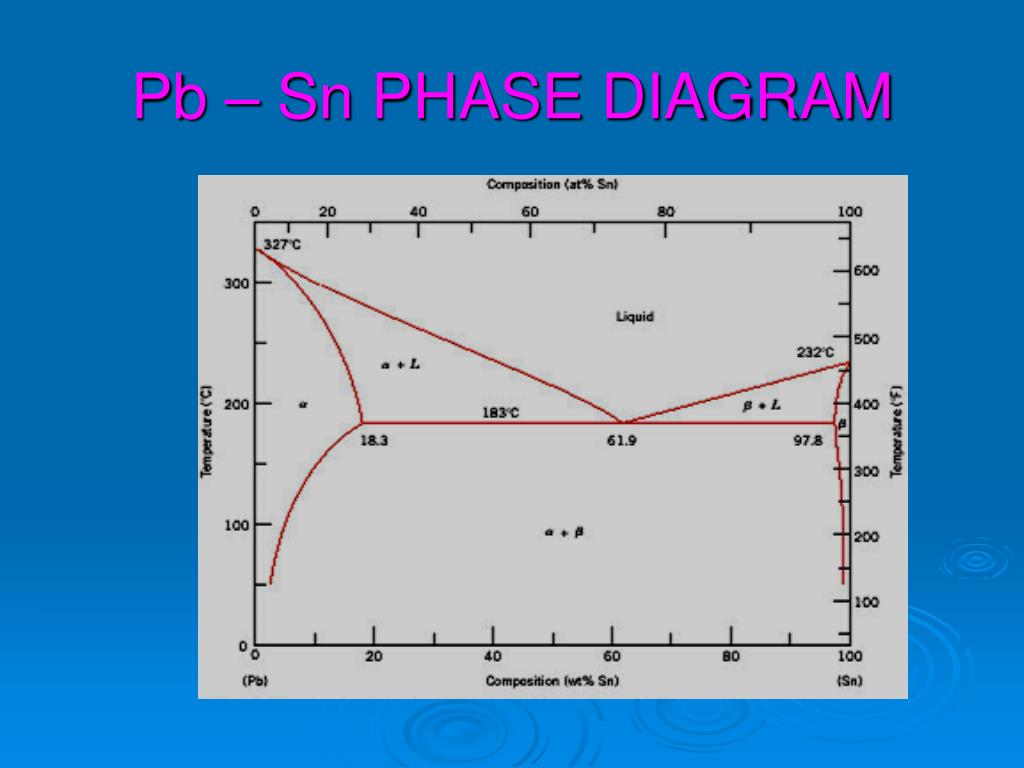
Pb-Sn phase diagram β phase: solid solution of Pb in tetragonal Sn α phase: solid solution of Sn in fcc Pb Liquid Pb (Fcc) Sn (Tetra) 0 50 100 150 200 250 300 350 0 10 20 30 40 50 60 70 80 90 100 T emperature Wt% The Pb-Sn system is characteristic of a valley in the middle. Such system is known as the Eutectic system. The central point is the.
Pb-sn phase diagram
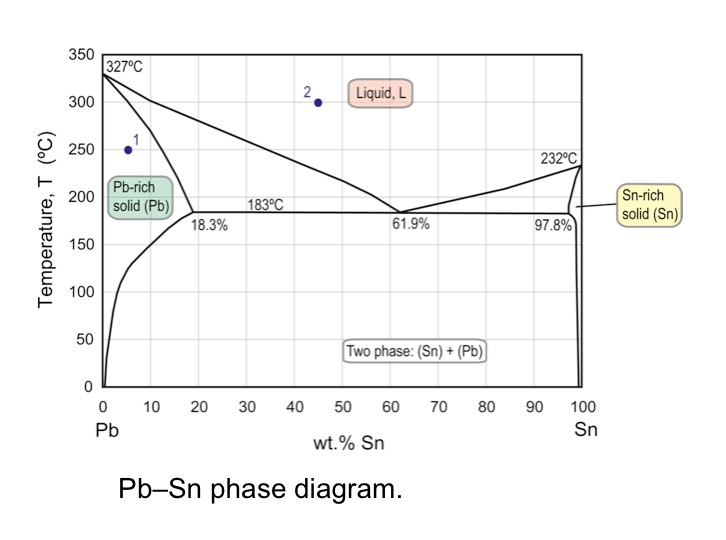
In the Pb-Sn phase diagram Pb is component A and Sn is component B. microstructural component. A component of the microstructure that has an identifiable characteristic morphology that developed due to its composition and thermal treatment. microstructure. (b) That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown below; the point labeled "B" represents the 75 wt% Sn-25 wt% Pb composition at 175°C. As may be noted, point B lies within the α + β phase field. A tie line has been constructed at 175°C; its intersection Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
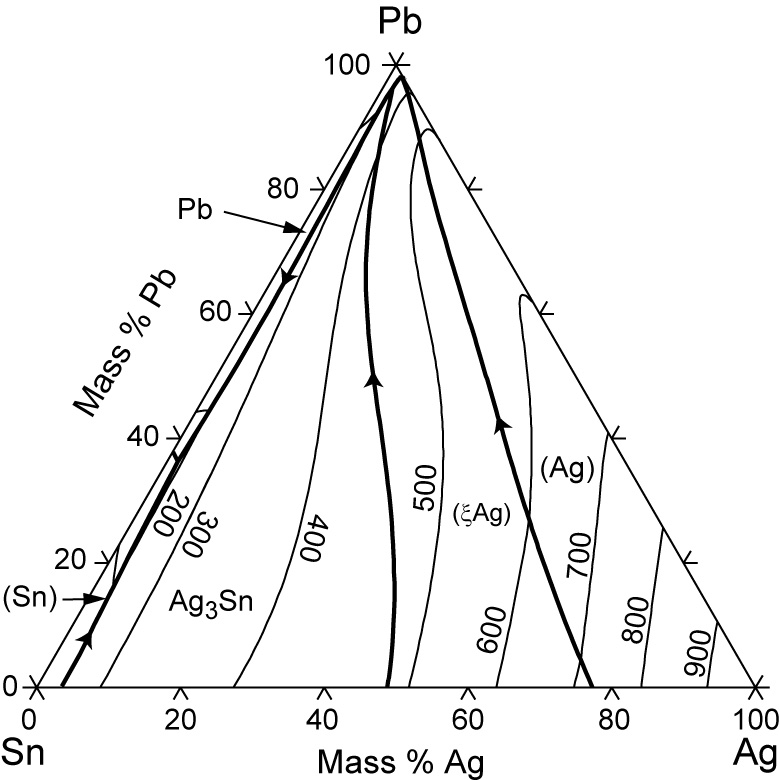
Pb-sn phase diagram. suggested as possible alternatives to Pb-Sn solders. 19 Figure 6 shows the effect of the addition of Ag, Bi, Sb, or Zn on the phase constitution of Sn-20mass%In alloys. a Fig. 3. Isothermal section diagrams of the Sn-In-Bi system at (a) 100°C and (b) 200°C. a Fig. 4. Isothermal section diagrams of the Sn-In-Sb system at (a) 100°C and (b) 200. The binary Pb-Sn phase diagram has been studied for over 100 years and is a classic eutectic. Lead (Pb) has an atomic number of 82 and a high density. Its crystal structure is face-centered cubic (fcc). At 50 C, 2% Sn is soluble in Pb and at the eutectic temperature, (b) That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown below; the point labeled "B" represents the 75 wt% Sn-25 wt% Pb composition at 175°C. As may be noted, point B lies within the α + β phase field. A tie line has been constructed at 175°C; its intersection The relation between the eutectic phase diagram and the g-plots for several temperatures is shown in Fig. 2.13. First we will repeat some basics about labeling phases. As usual homogeneous phases are labeled by Greek characters. Here α is a Pb rich phase with a small amount of solved Sn.
#modimechanicalengineeringtutorials, #mechanicalmagicmechanicallearningtutorials,Welcome to My YouTube Channel MODI MECHANICAL ENGINEERING TUTORIALS.This ch... represented in the portion of the Pb-Sn phase diagram shown below (at point B). Furthermore, the compositions of the phases, as determined from the tie line are Cα = 16 wt% Sn-84 wt% Pb Cβ = 97 wt% Sn-3 wt% Pb Inasmuch as the composition of the alloy C0 = 75 wt% Sn, application of the appropriate lever rule expressions (for Bi-Pb-Sn Vertical Section of Ternary Phase Diagram Cite this page. Citation Phase diagram available to subscribers; If you are having trouble in accessing SpringerMaterials remotely during the COVID-19 pandemic, Get help with remote access; To view content, please. This video explains the Pb-Sn phase diagramFor further reading: https://www.physicsforums /threads/sn-pb-phase-diagram.281790/
Below is the binary phase diagram for Pb-Sn. At 46.5 wt% Sn, what is the total weight fraction of a (alpha) phase just below the eutectic solidification temperature? 400 |327°C 300 Pb-80%Sn Pb-61.9%Sn 231°C Temperature,[°C] 200 F (Pb) 183°C 19 61.9 97.5 Sn 100 0 1 1 1 0 10 20 50 70 80 90 100 30 40 60 Pb-Sn[wt%] 0.65 Download scientific diagram | Pb-Sn phase diagram. from publication: Partial melting of a Pb-Sn mushy layer due to heating from above, and implications for regional melting of Earth's. The thermodynamic description of Sn-Bi-Pb from Yoon and Lee, Calphad 22 (1998) 167 was used for the present calculations. The liquid concentration paths for the alloys, Sn-.04Bi-.06Pb and Sn-.49Bi-.06Pb, are shown together with the liquidus projection of Sn-Bi-Pb system. The (Sn) phase is the primary phase in all cases. Figure 1 shows the Pb-Sn phase diagram and the composition for present experiments, in which it is very clear that the eutectic temperature is 183 • C, and the liquid-solid transition.
Question. : Question 3 Using the binary phase diagram below, explain what happens as the Pb-Sn compound with 70% weight of Pb cools slowly from 350*C to room temperature. 350 Melting point of pure Pb 327°C 300 Liquid, L Melting point of pure Sn 250 Eutectic point 232°C Liquidus Solidus L+ (Pb) Pb-rich solid (Pb) 183°C 18.3% 200 Temperature.
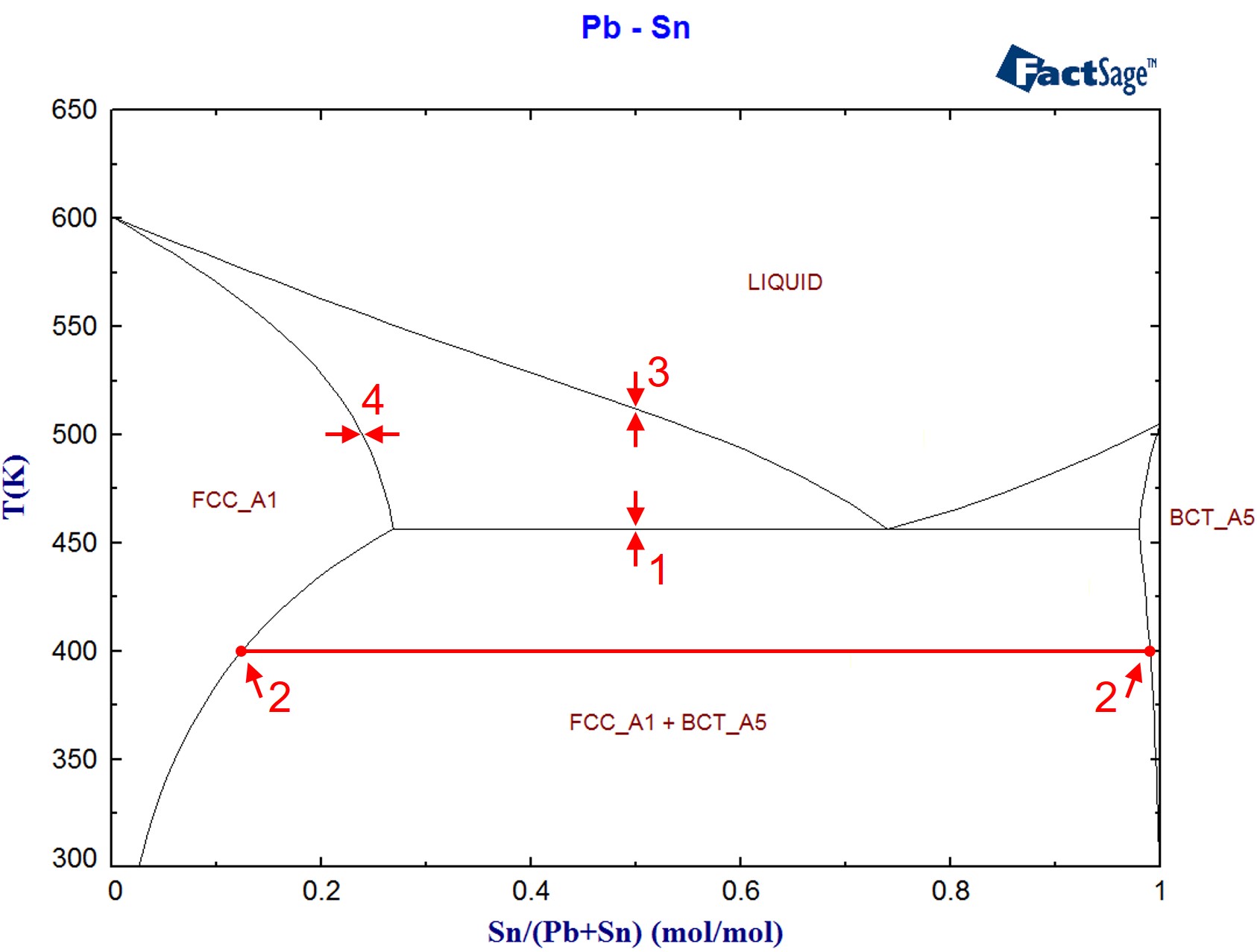
Phase Struktur-bericht Symbol Common Names Prototype Spacegroup Model * Liquid: n/a: L, L 1, L 2: n/a: n/a (Bi,Cu,Pb) 1 : Fcc: A1 (Cu), (Pb) Cu: Fm-3m (Cu,Pb,Sn) 1 (Va) 1: Bcc: A2 (beta Cu), beta: W: Im-3m (Cu,Sn) 1 (Va) 3: Bct: A5 (Sn), (beta Sn) beta Sn: I4 1 /amd (Cu,Pb,Sn) 1 : Cu 3 Sn.h: D0 3: gamma: BiF 3: Fm-3m (Cu,Sn) 0.75 (Cu,Sn) 0.25.
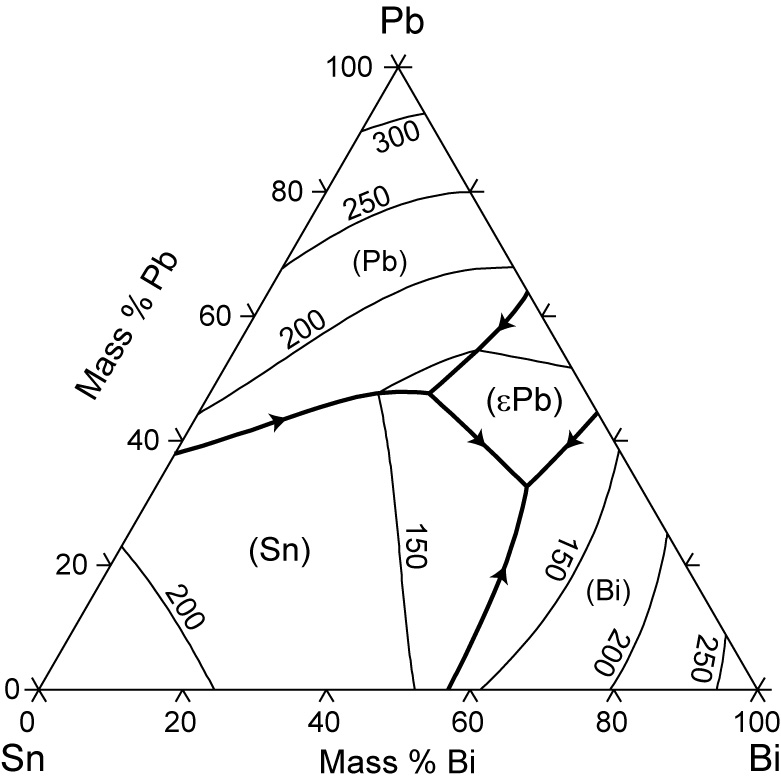
Sn-Bi-Pb equilibrium phase diagram. For the Lever calcula-tion, complete mixing in the liquid and complete diffusion in † In this paper, the percentage presents mass fraction, which is the same as weight fraction. ‡ The changeover to Pb-free solder technology requires changes in pre-tinning operations as well as solder.
simple but useful binary phase diagram for the purpose of understanding the Eutectic concept is that for Lead (Pb) and Tin (Sn). Pb-Sn Equilibrium Binary Phase Diagram The important point here is marked 61.9 and this is the Eutecic Point for the Pb-Sn Phase Diagram. It can be seen that with a composition of 61.9% Sn (Tin) and 38.1% Pb (Lead), the
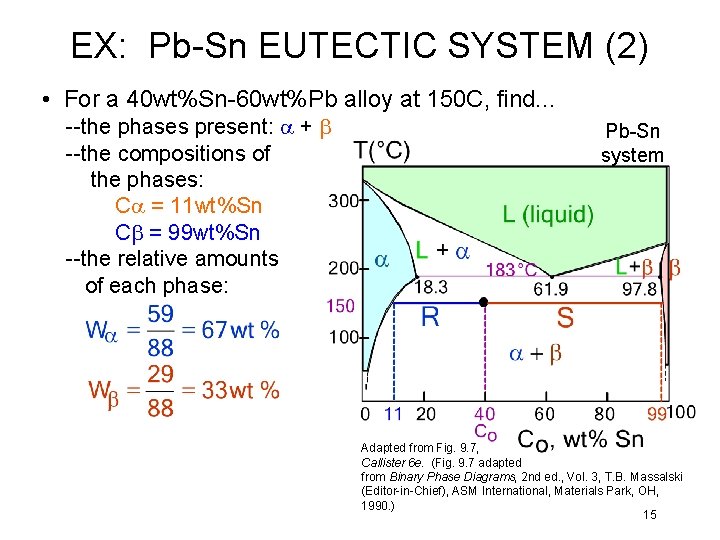
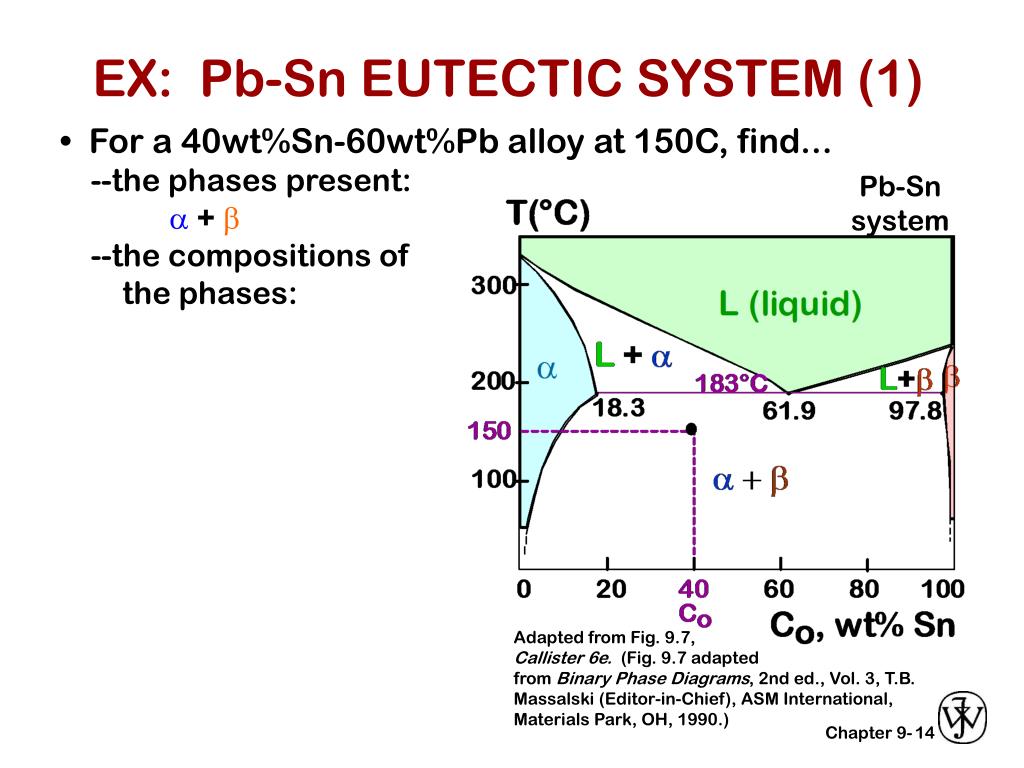
The tin-bismuth phase diagram Composition of the Bi-Sn soldier: 57wt% Bi Questions: 1. Give the names for the single phases in the Sn-Bi system 2. To fíll the name for each phase region 3. The eutectic. Pb-Sn system EX: Pb-Sn Eutectic System (1) a+ b--compositions of phases: C O = 40 wt% Sn--the relative amount of each phase: 150 40 Co 11...
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
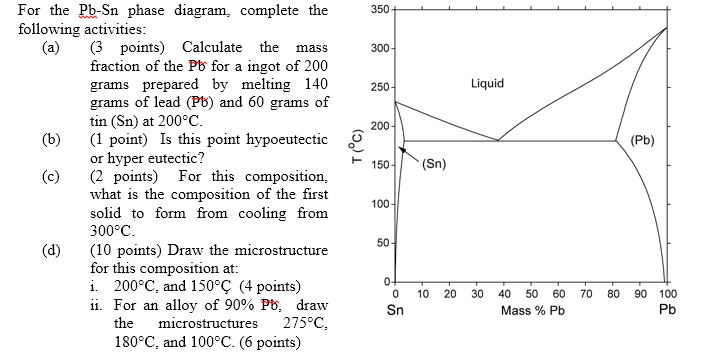
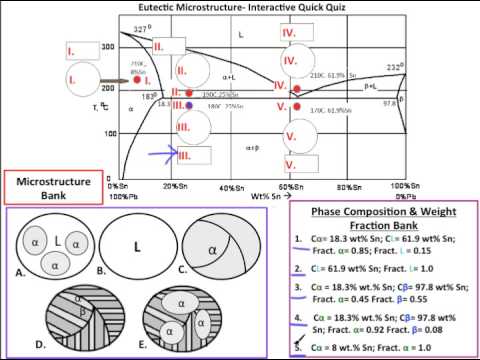
9.38 On the basis of the photomicrograph (i.e., the relative amounts of the microconstituents) for the lead- tin alloy shown in Figure 9.17 and the Pb-Sn phase diagram (Figure 9.8), estimate the composition of the alloy, and then compare this estimate with the composition given in the figure legend of Figure 9.17.
A phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically-distinct phases; or to show what phases are present in the material system at various T, p, and compositions. • Example: cooling 60%Pb - 40%Sn system
The Attempt at a Solution. It is probably an easy question, but I thought it is a good idea to consult first. a) At 183 C, the first liquid phase forms. b) We can draw a tie line and the point intersects with the liquidus line, gives us the composition of liquid. It is 61.9 wt % Sn. c) It is around 250 C. Because phase diagram is on liquidus line.
Eutectic 1. We have examined isomorphous phase diagrams, and used the example of the Cu-Ni phase diagram. In this module we will examine eutectic phase diagrams. A eutectic system has two components, and they have limited solubility. Upon cooling, the liquid will transform into two mixed solid phases. We will use the Pb-Sn phase diagram as an.
โลหะผสมมีส่วนผสม 75 wt% Sn - 25 wt% Pb ณ อุณหภูมิ 150oC จงหาว่า (a) มีเฟส (phase) ใดปรากฎบ้าง (b) เฟสที่ปรากฎนั นมีส่วนผสมทางเคมีเท่าใหร่
Example using the Pb-Sn Phase Diagram. Consider a 40 wt% Sn-60 wt% Pb alloy on the lead-tin phase diagram. Part 1: At 183.1 degrees C, just above the eutectic line, a) what phase(s) is (are) present? b) what is (are) the compositions of the phase(s)? c) what is the relative amount of each phase present, in mass fraction?
In the Pb-Sn phase diagram Pb is component A and Sn is component B. microstructural component. A component of the microstructure that has an identifiable characteristic morphology that developed due to its composition and thermal treatment. microstructure.
In the Pb-Sn phase diagram Pb is component A and Sn is component B. Microstructural Component. A component of the microstructure that has an identifiable characteristic morphology that developed due to its composition and thermal treatment. Possible microstructural components on the Pb-Sn phase diagram include alpha and beta in the single phase.
Created Date: 11/3/2015 10:28:58 AM





















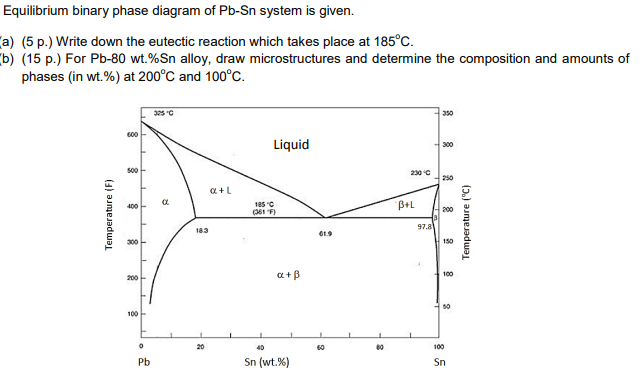


0 Response to "43 Pb-sn Phase Diagram"
Post a Comment