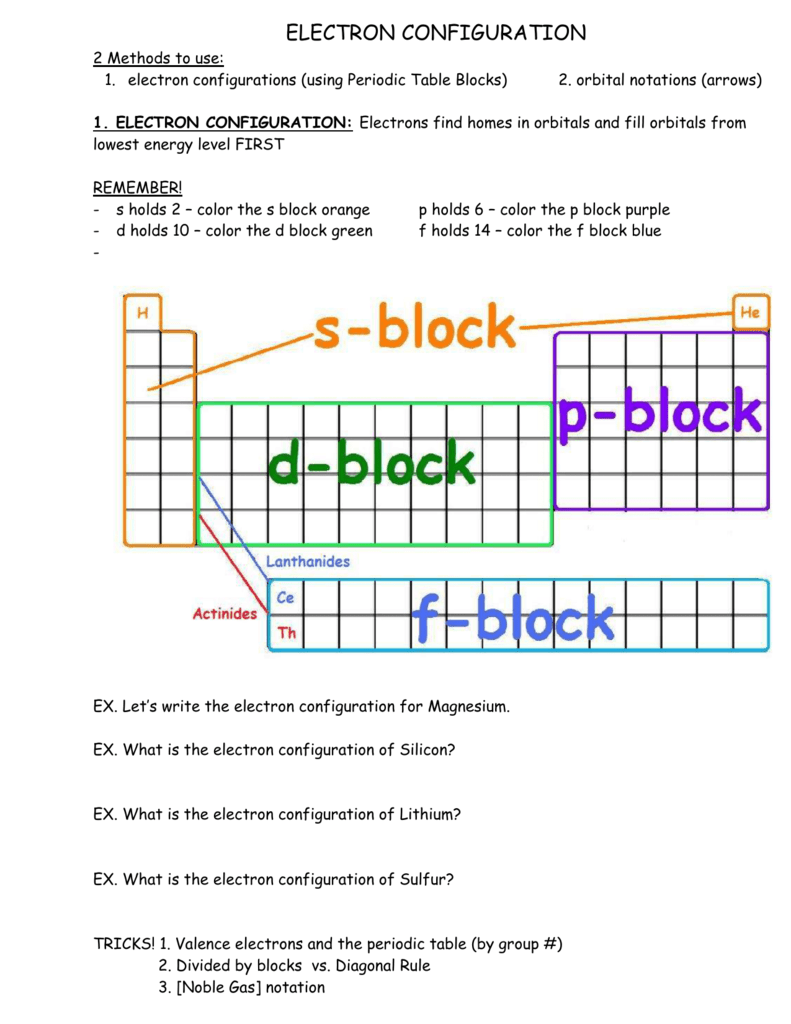
43 Orbital Diagram For Sulfur
Gravity. Mg (Magnesium) Click card to see definition 👆. Tap card to see definition 👆. What element is represented by this orbital diagram? Click again to see term 👆. Tap again to see term 👆. S (Sulfur) Click card to see definition 👆. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Mar 23, · Show the orbital-filling diagram for (sulfur). Stack the subshells in orderof energy, with the lowest-energy sub shell at the bottom and thehighest-energy subshell at the top. Show the orbital-filling diagram for (bromine).Status.
Answer and Explanation: 1. Sulfur is an element that belongs to the p-block. The atomic number of sulfur is 16, so contains 16 electrons arranged in increasing order of energy orbitals. Among all.
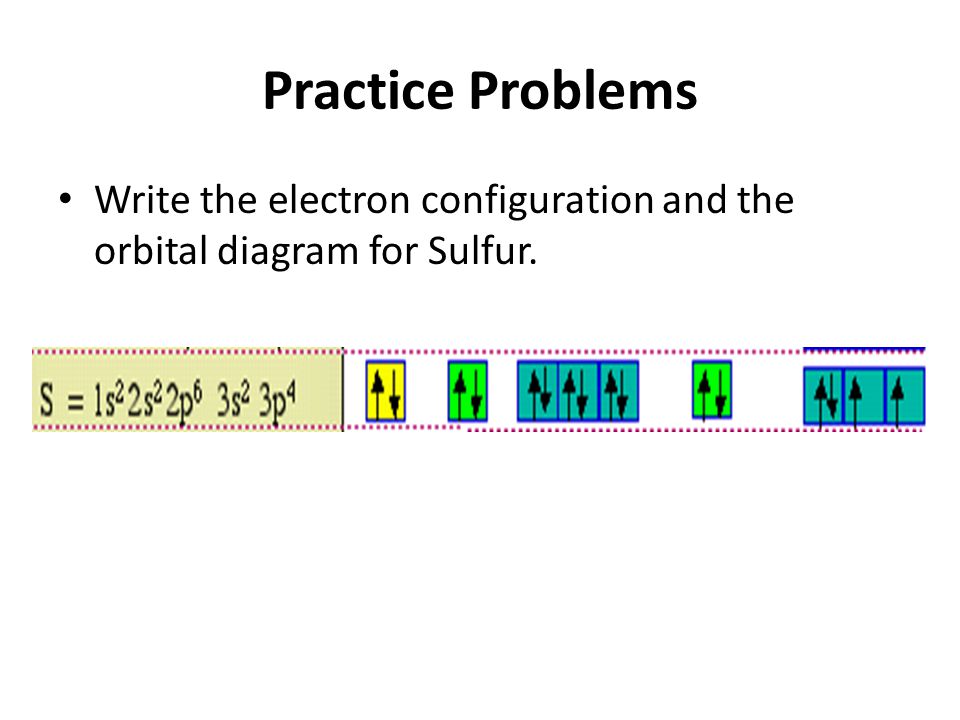
Orbital diagram for sulfur
Answer (1 of 2): Sulphur, a pretty yellowish dust-like looking element has an electronic configuration according to Bohr Bury Rule is 2, 8, 6. According to Afbau Principle, it has electronic config. is : 1 s^2 2 s^2 2 p^6 3 s^2 3 p^4 When it forms a molecule, it does so with 8 sulphur atom... Sulfur atoms have 16 electrons and the shell structure is 2.8. 6. The ground state electron configuration of ground state gaseous neutral sulfur is [Ne]. 3s 2. Just so, what is the orbital diagram for silicon? Silicon has 14 electrons in the following orbital configuration 1s2 2s2 2p6 3s2 3p2 when neutral in charge. Also the crystalline form is. The orbital diagram shows the valence electrons of sulfur, which has 16 electrons. If the electrons were added to the atom one at a time, which would be - 18770841
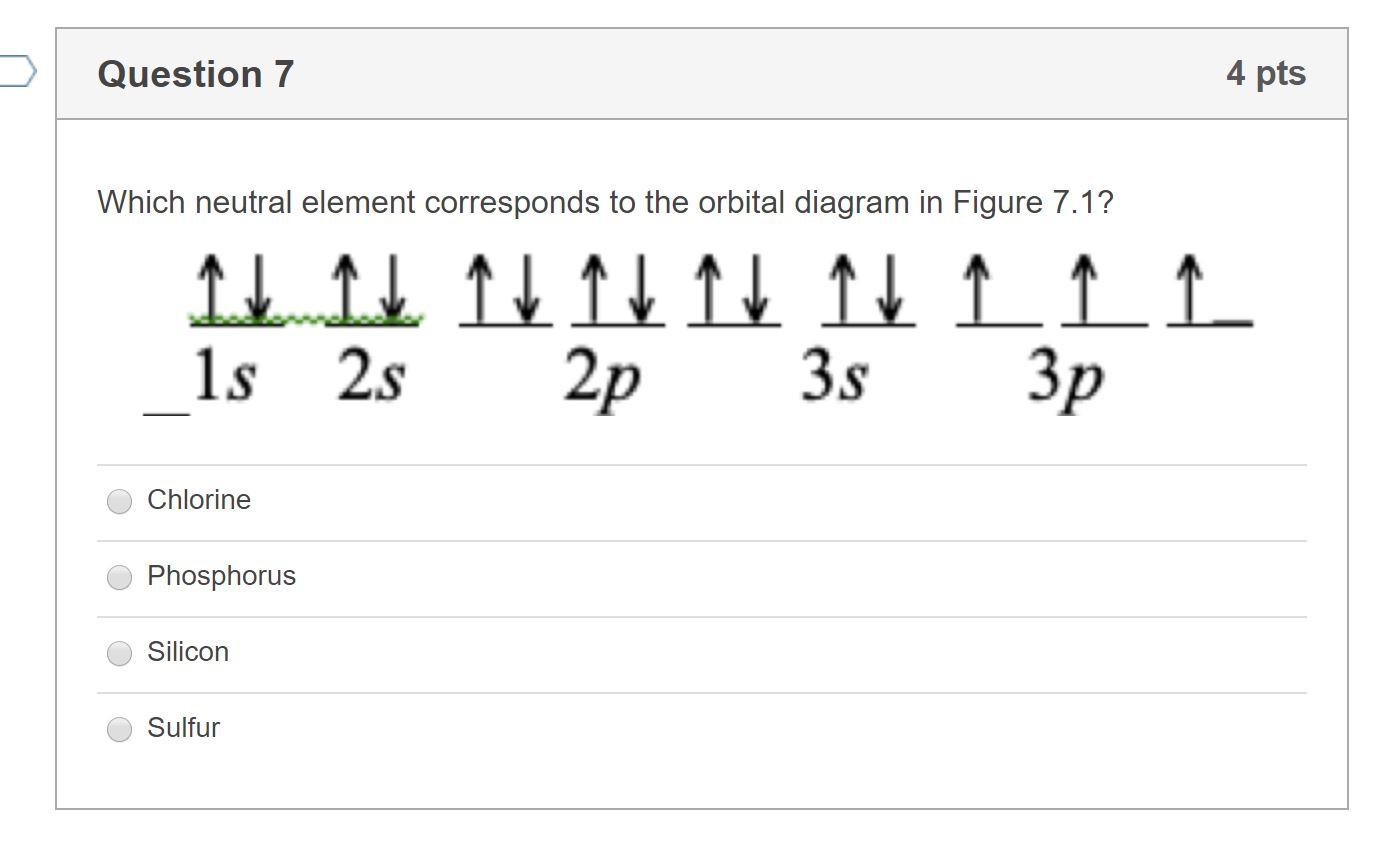
Orbital diagram for sulfur. Sulphur/Sulfur (S) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. 14+ Orbital Diagram For Sulfur. Click within the orbital to add electrons. The aufbau principle tells us that the first energy level (k shell) containing the 1s orbital was completed with the last sulfur : Write the electron configurations for cobalt and lead. Combine the two sodium valence atomic a sulfur has a ne3s23p4 valence electron. Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
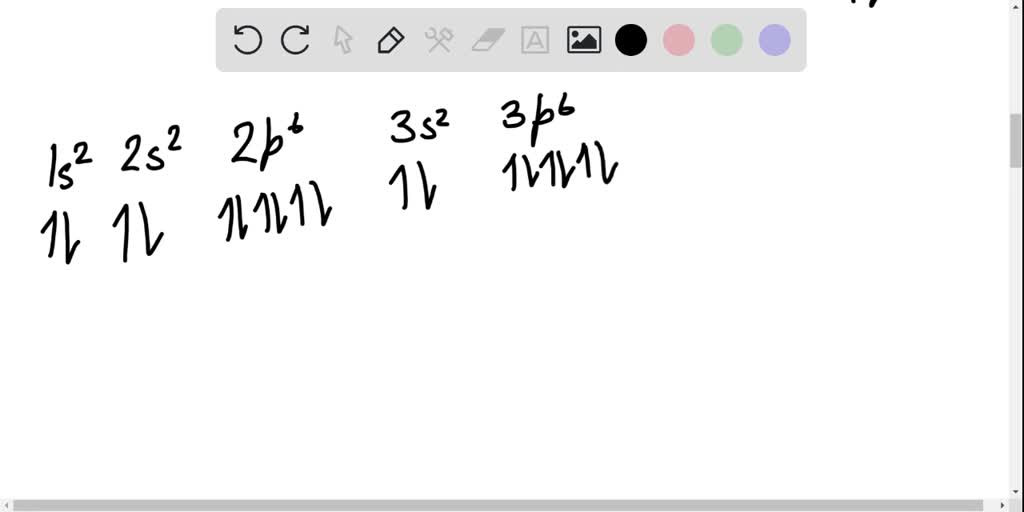
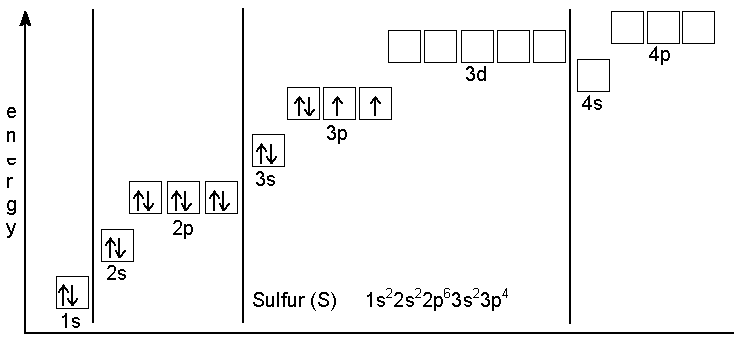
Atomic number of Sulfur = 16. Electronic configuration of S: 1s2, 2s2, 2p6 and 2s2 are totally filled. only 3p^4 is partially filled. p sub shell has three orbital. As per Hund's rule electrons in the orbital of sub shell fill in such a way that orbitals are first singly occupied and after that pairing occurs. Answer (1 of 2): Sulphur, a pretty yellowish dust-like looking element has an electronic configuration according to Bohr Bury Rule is 2, 8, 6. According to Afbau Principle, it has electronic config. is : 1 s^2 2 s^2 2 p^6 3 s^2 3 p^4 When it forms a molecule, it does so with 8 sulphur atom... In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two. Chemistry. Chemistry questions and answers. Choose the orbital diagram for sulfur. 11 11 11 111111 2p 11 3p ls 2s 3s 11 O 11 11 1s 2s 11 11 11 2p 11 3p 3s 1L 11 11 2s 11 11 11 2p 1 ls 3s 3p о 11 ls 11 2s 11 11 | 11 | 11 2p 1111 3s 3p Submit Request Answer.
Sulfur atoms have 16 electrons and the shell structure is 2.8. 6. The ground state electron configuration of ground state gaseous neutral sulfur is [Ne]. 3s 2. Just so, what is the orbital diagram for silicon? Silicon has 14 electrons in the following orbital configuration 1s2 2s2 2p6 3s2 3p2 when neutral in charge. Also the crystalline form is. The orbital notation for sulfur is: Each arrow represents an electron. We start filling out the chart at the 1s orbital and work upwards, which is the Aufbau principle. When we get to the p. Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is ‘Na’. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles. Answer (1 of 2): Sulphur, a pretty yellowish dust-like looking element has an electronic configuration according to Bohr Bury Rule is 2, 8, 6. According to Afbau Principle, it has electronic config. is : 1 s^2 2 s^2 2 p^6 3 s^2 3 p^4 When it forms a molecule, it does so with 8 sulphur atom...
Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen.
Problem: Write the electronic configurations of the six cations that form from sulfur by the loss of one to six electrons. For those cations that have unpaired electrons, write orbital diagrams.S+. FREE Expert Solution. We're being asked to determine the electron configuration and orbital diagram (if there is an unpaired orbital) for S +.
The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals.
What is the orbital diagram for the atom Sulfur? close. Start your trial now! First week only $4.99! arrow_forward. Question. What is the orbital diagram for the atom Sulfur? check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here.
The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur. High pressure solid allotropes. In a high-pressure study at ambient temperatures, four new solid forms, termed II, III, IV, V have been characterized, where α-sulfur is form I. Solid forms II and III are polymeric, while IV and V are metallic (and are.
Orbital diagram. Tags: Question 8. SURVEY . 300 seconds . Q. What is the shorthand electron configuration for Sulfur atom? answer choices [Ar] 3p 4 [He] 3s 2 3p 4 [Ne] 3s 2 3p 4 [Na] 3s 2 3p 3. Tags: Question 9 . SURVEY . 30 seconds . Q. What is the noble gas shorthand electron for Sulfur atom? answer choices [Ar] 3p 4 [He] 3s 2 3p 4 [Ne] 3s 2.
The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Nov 14, · Draw an orbital diagram for boron. Draw an orbital diagram for scandium Show the orbital-filling diagram for N (nitrogen).
The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The boxes represent sulfurs orbitals. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom there are 16 electrons.
Orbital Filling Diagram for Sulfur. what is the orbital diagram for sulfur the orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each the arrows solved show the orbital filling diagram for s sulfur st answer to show the orbital filling diagram for s sulfur stack the sub shells in order of energy with the.
The orbital diagram shows the valence electrons of sulfur, which has 16 electrons. If the electrons were added to the atom one at a time, which would be - 18770841
This time we have collected a handy of orbital diagrams with various types in high definition! There are alkali, argon, electron, nitrogen, phosphorus, silicon, and sulfur orbital diagrams that you can save for free. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams.
This adds up to the explanation of the molecular orbital diagram of SO2. Similarities between Sulfur and Oxygen atoms. Both O and S have the same outer electric configuration of ns2 and np4. O and S are usually divalent. O and S are non-metals. Both exhibit allopatric form. In reaction with metals, both react with the oxidation state of -2.
The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.. The bond order in sulfur dioxide, for example, is 1.5 the average of an S-O single bond in one Lewis structure and an S=O double bond in the other.
3D model to visualise the shapes of atomic orbitals. s, p and d.
SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of 70.062 g/mol, this compound is made up of one Sulfur atom and two Fluoride atoms. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or.
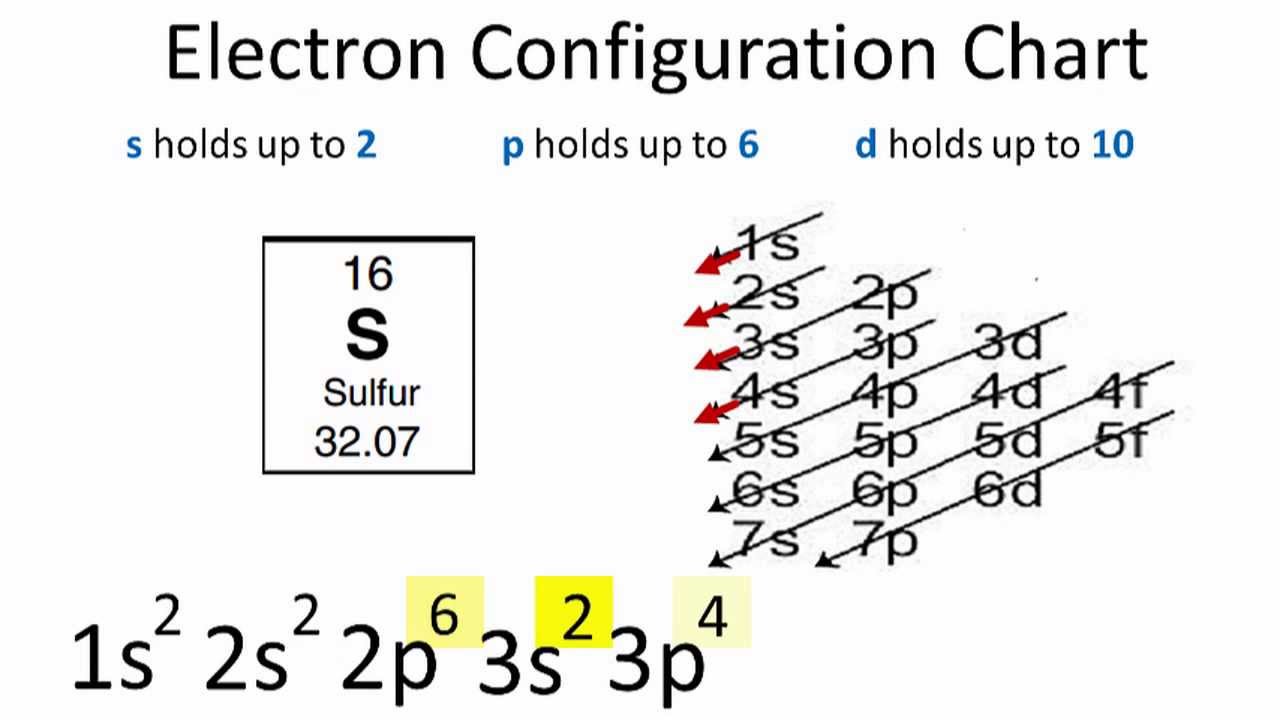
The electron configuration for sulfur is 1s 2 2s 2 2p 6 3s2 3p4 and can be represented using the orbital diagram below. May 30, 2020 How many orbitals does sulfur have?
To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.http://scientifictutor /
The fluorine atoms are sp 3 hybridized (3 lone pairs and one bonding pair), and the overlap of each sp 3 orbital on fluorine with a dsp 3 orbital on sulfur will form a s bond. For compounds, like SF 6, which require six equivalent molecular orbitals, mix six atomic orbitals, s + p + p + p + d + d.
The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The electron configuration for hydrogen is 1s1 and the orbital notation is a circle with one slash through it like. Stack the subshells in orderof energy with the lowest energy sub shell at the bottom.
How do you write the orbital diagram for sulfur? Chemistry Quantum Mechanical Model of the Atom Orbitals, and Probability Patterns. 1 Answer anor277 Aug 21, 2017 Well.
In the figure below, an atomic orbital diagram is used to illustrate the order of filling for the first ten electrons as shown by the numbers entered in the boxes. As an example, consider the electronic structure of sulfur. Since sulfur has 16 electrons, its electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4
SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38.
orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z and 4s orbital, and only 1 electron occupies each of the 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule.
The correct orbital diagram of this element sulfur? We can't make diagrams on answers . Sulphur's configuration is 2, 8, 6. You may either use this information or refer to the element's page on.
This problem has been solved! Draw the orbital energy diagram for Sulfur. What would the electron configuration of the S2+ cation be? From which orbital did you remove electrons? Who are the experts? Experts are tested by Chegg as specialists in their subject area.



























0 Response to "43 Orbital Diagram For Sulfur"
Post a Comment