43 Electron Dot Diagram For H2o
Follow these steps to draw the lewis dot structure for H2S. Step 1: In the first step, determine the total valence electron present in H2S. As we know hydrogen only has one valence electron in its last shell and Sulfur belongs to the 16th group in the periodic table so it contains 6 electrons in its last shell. (e) The complete Lewis electron dot diagram of methanal (formaldehyde) is shown in the box below. Molecules of methanal can form hydrogen bonds with water. In the box below, draw a water molecule in a correct orientation to illustrate a hydrogen bond between a molecule of water and the molecule of methanal. Use a
The electron-dot structure of H 2 O is:. The oxygen atom shares its one electron with each hydrogen atom to form a covalent bond in the water molecule.
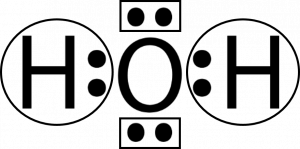
Electron dot diagram for h2o
I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle. Students will use a Venn diagram to compare lightning and static electricity. Then, students will experiment with static electricity and read nonfiction passages about lightning and lightning rods. Finally, they will apply their learning to construct a model of a lightning rod system that protects a house from a lightning-induced fire. The total valence electron is available for drawing the Sodium chloride (NaCl) lewis structure is 8. NaCl is a face-centered cubic unit cell that has four cations and four anions. In the NaCl lewis dot structure, the sodium atom completes its octet by transferring one electron to the chlorine atom.
Electron dot diagram for h2o. For example, a hydrogen molecule, H 2, forms when two hydrogen atoms each share their outer electron. A dot and cross diagram to show the bonding in hydrogen. An ammonia molecule,. Vsepr lab answer key. 4 g of mercury from 37. it PhET- Density Activity- Funsheet April 21st, 2019 - Phet Waves On A String Answer Key Phet Wave on a String Simulation In this simulation you will investigate the properties of waves and how changing Related searches for phet waves on a string answer key Wave on a String Simulation PhET Wave Newman H2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. H2O2 is a chemical compound with the IUPAC name Hydrogen Peroxide. It is the simplest peroxide compound, i.e., a molecule containing an Oxygen-Oxygen single bond. It is a pale blue liquid in its standard state and slowly reacts with sunlight and decomposes into water and oxygen. Therefore, corresponding to the question, we can that the correct Lewis dot structure of water molecules is number 3. So, Option C is the correct answer. Note: (1) Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of an atom within a molecule.
The diagram opposite shows the lewis structure for the water molecule, h2o, and the ethene molecule, c2h4. 10+ C2H6O Lewis Structure. • for main group elements, the number of valence e − is equal to the group number. The structure on the right is the lewis electron structure, or lewis structure, for h2o. Feb 25, 2020 · SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ]. What is the lewis structure for hs2? Like a molecule of water, hydrogen sulfide has a bent geometric structure with a bond angle of 92.1° and bond lengths of 136 picometers (1. Formula for determining composition of hydrates. The lewis structure of hydrogen sulfide is easy to draw and understand. The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. In order to determine the molecular geometry for H2O observe the Lewis structure of the same. I quickly take you through how to draw the Lewis Structure of water H2O. COVID-19 is an emerging rapidly evolving situation.
The electron configuration of an element is 1s22s2.. (H2O) is 100 °C.... A Lewis dot diagram should contain one calcium atom and one oxygen atom to show how ... I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle. Key Points To Consider When Drawing The SO3 Electron Dot Structure. A three-step approach for drawing the SO3 Lewis structure can be used. The first step is to sketch the Lewis structure of the SO3 molecule, to add valence electrons around the sulfur atom; the second step is to add valence electrons to the three oxygen atoms, and the final step is to combine the step1 and step2 to get the SO3. Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3. Secondly, what is the shape of nh3.
Nov 17, 2021 · It is explained with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory, which says the presence of a lone pair on the nitrogen atom makes the complete structure of NH3 bent giving a bond angle of 107°. It might surprise you that the ideal bond angle for the bent geometrical diagram is 109.5°.
H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure.This "bent" molecular structure gives it many unique properties such as being polar.One of the most fascinating phenomena is the idea of "hydrogen bonding.
In this manner, what is the electron dot structure of h2o? Drawing the Lewis Structure for H 2 O Another straight forward Lewis structure. You have a total of 8 valence electrons available to fill the octets of Oxygen and Hydrogen. Remember that Hydrogen only needs two electrons to have a full outer shell.
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc...
Lewis Structure of H 2 O (Water) - Drawing Steps. Lewis structure of water molecule contains two single bonds around oxygen atom. number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Each step of drawing lewis structure of H 2 O are explained in this tutorial.
Draw the electron dot diagram for the CH_3^- molecule and determine its VSEPR shape. View Answer.. H2O c. CCl4. View Answer. List any electron pair arrangement(s) that result in molecules ...
Electron dot diagram for h2o. Lewis structures and the shapes of molecules. Locate the element you are drawing an electron dot diagram for on the periodic table of elements. Lewis dot structure of h2o water. A lewis structure is a type of shorthand notation. But we have two of them so lets multiply that by 2.
The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. Moreover, these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O molecule. It is the reason why the bond angle that should have.
Answer (1 of 2): Firstly you need to know the number of electrons present in outermost shell of O and H atom O: 1s2 2s2 2p4 There are six electrons in outermost shell H: 1s1 there is one electron in outermost shell Write down the symbol of atom and no. of electrons in the outermost shell are r...
The lewis dot structure of OF2 is very easy to draw if you follow the simple approach of drawing the lewis diagram. There is a total of 16 lone pair electrons and 4 bonded pair electrons present in the OF2 lewis structure. Steps to follow for drawing the OF2/F2O lewis structure. 1..
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Lewis diagram for the ether c2h5oc2h5
Nov 15, 2021 · Step 3: Sketch the Skeletal Diagram of the Molecule. In Lewis Structure, we use atomic symbols like C for carbon, H for hydrogen to represent the constituent atoms, and electron dot notation to represent the valence shell electrons. Let us look at the below skeletal sketch: The atomic symbols: Atomic symbols along with dot notations:
The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms and its electrons. In H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms.
NO DOTS Draw the e- dot diagram for the – ion COMPLETE outer shell Step 3 Enclose both in brackets and show each charge Draw the Lewis Diagrams LiF MgO CaCl2 K2S Drawing molecules using Lewis Dot Structures Symbol represents the KERNEL of the atom (nucleus and inner e-) dots represent valence e- Always remember atoms are trying to complete.
Let's do the Lewis structure for water: H2O. On the periodic table, Hydrogen's in group 1, it has 1 valence electron; but we have two of them, so let's multiply that by 2. And Oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. So 1 times 2 is 2, plus 6; 2 plus 6 equals 8. We have a total of eight valence electrons.
To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. These valence electrons are represented by drawing dots around the individual atoms, hence the Lewis dot structure.
Nov 06, 2021 · The shape is like a swing. The Lewis structure and electron-domain geometry of SF. Keep in mind the exceptions to the Octet Rule!!! N02 2. g. AICf3 15. (i) Draw the complete Lewis electron-dot structure for each molecule. , 1,3,2) where 1 indicates the absence of a coefficient. About Becl2 Lewis Structure.
Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get.
Lewis structure diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion Lewis symbol symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair two (a pair of) valence electrons that are not used to form a covalent bond octet rule
12+ Electron Dot Structure Of H2O. Electrons of the covalent bond by two dots. In order to eject an electron from a metal, a photon of a certain minimum energy must strike the sur. There are two hydrogens that bind with 1 electron from those 6, so there are 2 electrons that are binding with hydrogen. Know how and when to incorporate double and.
Correct option is. C. Oxygen atom has 6 valence electrons , out of which 2 are involved in bonding with the 2 hydrogen atoms. Therefore water molecule has 2 bonded pair of electrons and 2 lone pair (non bonded) pair of electrons and the correct electronic structure is (C).
Which Electron Dot Diagram Represents H2. The left diagram shows a Lewis dot structure of sodium with formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed. We can use Lewis dot formulas to show covalent bond formation. 1. H. 2 molecule. +.
H 2 O Lewis Structure Video. Video Transcript: Here, we're going to do a dot structure for water, H2O. Let's write that down: H2O. What we want to find out first is how many valence electrons does water have. I'm counting all the outer shell electrons. I'll need my periodic table.
Determine the theoretical yield of HCl if 60.0 g of BCl3 and 37.5 g of H2O are reacted according to the following balanced reaction. A possibly useful molar mass is BCl3 = 117.16 g/mol. BCl3(g) + 3 H2O(l) → H3BO3(s) + 3 HCl(g)
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are.
The arrangement of valance electrons in atom can be representing by electron dot structure or Lewis structure. The diagrams which show the bonding between. diagramweb diagramweb Lewis_structure. This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond.
The total valence electron is available for drawing the Sodium chloride (NaCl) lewis structure is 8. NaCl is a face-centered cubic unit cell that has four cations and four anions. In the NaCl lewis dot structure, the sodium atom completes its octet by transferring one electron to the chlorine atom.
Nov 15, 2021 · Step 3: Draw the skeleton diagram of the molecule. We are now going to sketch the skeletal diagram of sulfate ion with the help of atomic symbols and dot line structures. Here, we have put the symbols of sulfur and oxygen as per notations and put the valence electrons as.
1 Answer. Ernest Z. Jul 15, 2014. You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom. The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell.
Students will use a Venn diagram to compare lightning and static electricity. Then, students will experiment with static electricity and read nonfiction passages about lightning and lightning rods. Finally, they will apply their learning to construct a model of a lightning rod system that protects a house from a lightning-induced fire.

0 Response to "43 Electron Dot Diagram For H2o"
Post a Comment