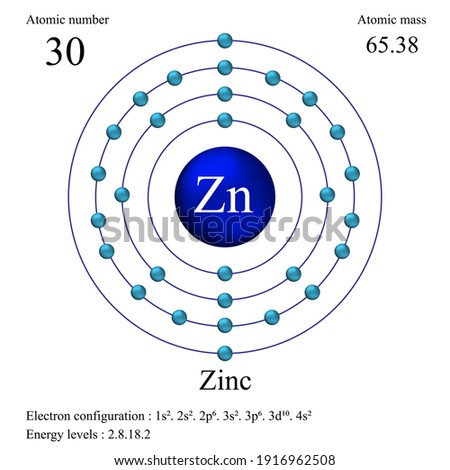
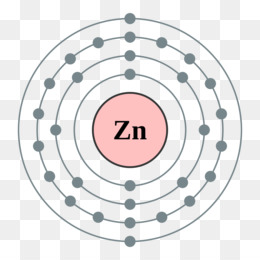
42 Lewis Dot Diagram For Zinc
Zinc nitride | Zn3N2 or N2Zn3 | CID 12130759 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities. The chemical structure for the compound could be given as below. Zinc Oxide Chemical Formula. Its presence could be felt in nature and taken as the largest mineral in New Jersey USA. The compound has a hexagonal crystalline structure and there is just plenty of process to synthesize the zinc oxide.
Ionic Bonding Lewis Dot Structures worksheet (S-C-4-2_Ionic Bonding Lewis Dot Structures.doc and S-C-4-2_Ionic Bonding Lewis Dot Structures KEY.doc) Periodic tables (one per student) helium-filled latex balloon. hydrogen-gas-filled latex balloon. zinc or aluminum (if H 2 gas tank is not available) 4-6M HCl (if H 2 gas tank is not available.

Lewis dot diagram for zinc
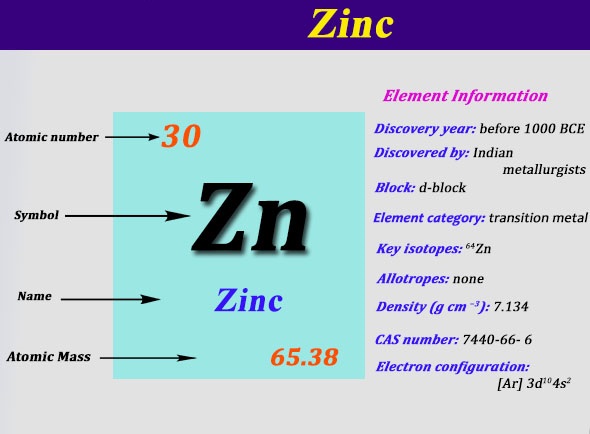
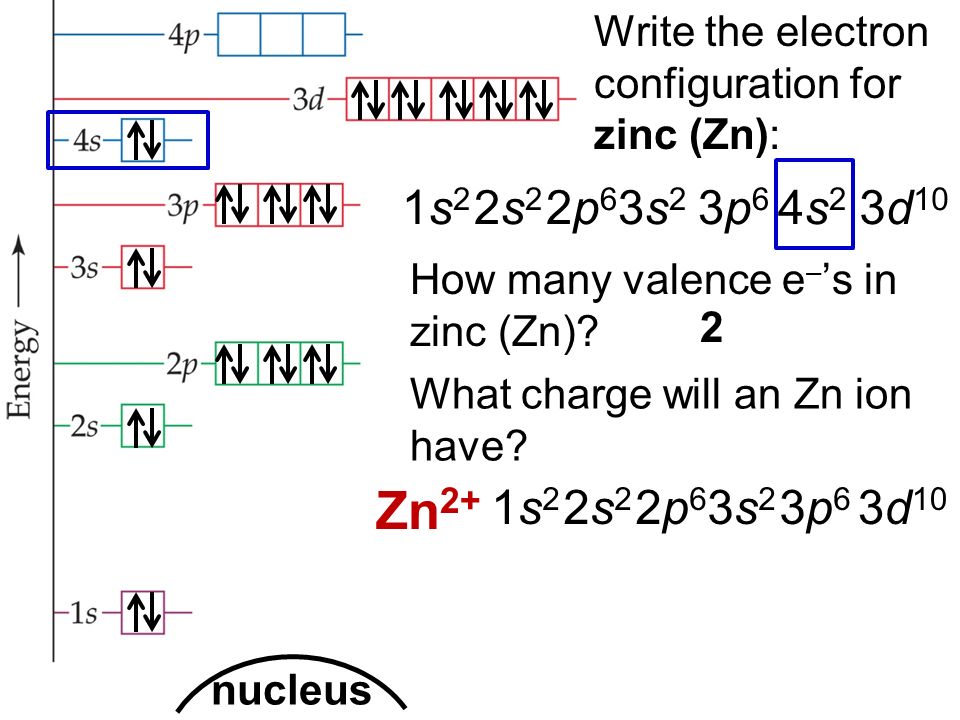
In zinc, these are the two 4s electrons, so in a Lewis structure zinc typically looks like Zn: 2. level 1. TangerineX. BS Comp Sci. 8 years ago. you typically don't make lewis dot structures of a singular atom. If you are looking for the Zn Chrystal, a lattice structure would be a better description of what the material looks like. The electron dot diagram for zinc is "Zn:" > Zinc (element number 30) is in the 4th Period of the Periodic Table. From left to right, you count two "4s" electrons and ten "3d" electrons. The "3d" shell is a filled inner shell, so only the "4s" electrons are valence electrons. Thus, the electron dot structure for zinc is "Zn:" Zinc 13. Carbon 14. Iodine 15. Oxygen 16. Barium 17. Aluminum 18. Hydrogen 19. Xenon 20. Copper. LEWIS DOT DIAGRAMS Name Lewis diagrams are a way to Indicate the number of valence electrons around an atom. are all examples of this type of diagram. Draw Lewis dot diagrams of the followlng atoms. carbon
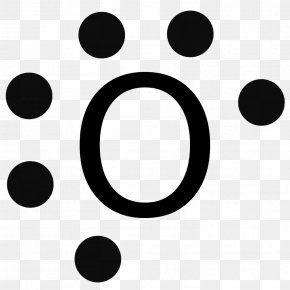
Lewis dot diagram for zinc. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons. TTUchme1010 teaches viewers how to draw the lewis dot structure for sulfate. The formula for this is SO4^2-. 2- means we will have to add 2 electrons into the lewis dot structure. First, we will have Sulfur in the middle with Oxygen surrounding it. Sulfur is in group 6A so it have 6 valence electrons and oxygen has six, so fill this all in around the elements. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. What does the Lewis Dot Structure of Zinc look like? wiki.answers › … › Elements and Compounds › Metal and Alloys How does the Lewis dot structure of zinc look like? Zinc is #30. It has an electron configuration of [Ar] 4s2, 3d10. The 4s2 electrons are the bonding
How does the Lewis dot structure of zinc look like? Zinc is #30. It has an electron configuration of [Ar] 4s2, 3d10. The 4s2 electrons are the bonding electrons. The dot structrue 2 dots. Zn: The electron dot, or Lewis dot diagram for xenon is the symbol Xe surrounded by four pairs of dots, representing eight valence electrons. Refer to the related link for an illu. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Structure, properties, spectra, suppliers and links for: Zinc iodide, 10139-47-6. Each dot diagram consists of; an elemental symbol, which represents the kernel of the atom, and a group of 1-8 dots which shows the configuration of the outermost electron shell of the atom, also called the valence shell… To make a Lewis dot diagram, you need to know how many electrons are in the valence shell." Materials
Zinc 13. Carbon 14. Iodine 15. Oxygen 16. Barium 17. Aluminum 18. Hydrogen 19. Xenon 20. Copper. LEWIS DOT DIAGRAMS Name Lewis diagrams are a way to Indicate the number of valence electrons around an atom. are all examples of this type of diagram. Draw Lewis dot diagrams of the followlng atoms. carbon The electron dot diagram for zinc is "Zn:" > Zinc (element number 30) is in the 4th Period of the Periodic Table. From left to right, you count two "4s" electrons and ten "3d" electrons. The "3d" shell is a filled inner shell, so only the "4s" electrons are valence electrons. Thus, the electron dot structure for zinc is "Zn:" The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE. Diagrams - Zinc. Bohr Diagram- Valence Electrons: 2. . Electron Configuration. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10. Noble Gas Configuration. Lewis Dot Diagram.
A step-by-step explanation of how to draw the Zn Lewis Dot Structure.For the Zn structure use the periodic table to find the total number of valence electron...
A step-by-step explanation of how to draw the ZnBr2 Lewis Dot Structure.For ZnBr2 we have an ionic compound and we need to take that into account when we dra...
LEWIS DOT DIAGRAMS Name Lewis diagrams are a way to Indicate the number of valence electrons aroun an atom. A\5Ö are all examples of this type of diagram. Draw Lewis dot diagrams of the following. l. calcium 2. potassiürn rgon 4. aluminum 2-3 -B 5, bromine 6. carbon . 7. helium 8. oxygen 9. phosphorus . hy rogen
Zinc is #30. It has an electron configuration of [Ar] 4s2, 3d10. The 4s2 electrons are the bonding electrons. The dot structrue 2 dots. Zn:
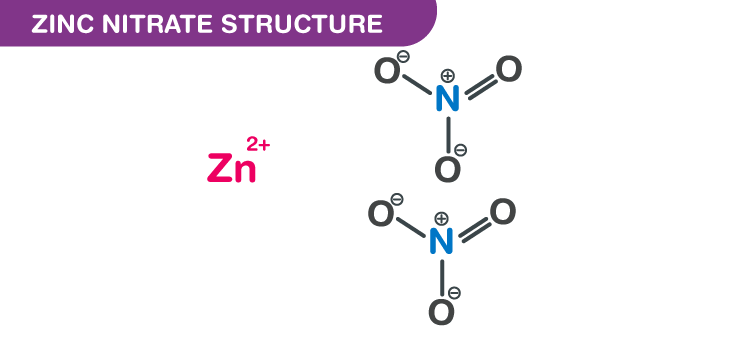
A step-by-step explanation of how to draw the Zn(NO3)2 Lewis Dot Structure.For Zn(NO3)2 we have an ionic compound and we need to take that into account when.
In zinc, these are the two 4s electrons, so in a Lewis structure zinc typically looks like Zn: 2. level 1. TangerineX. BS Comp Sci. 8 years ago. you typically don't make lewis dot structures of a singular atom. If you are looking for the Zn Chrystal, a lattice structure would be a better description of what the material looks like.
Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Exercises. 1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. 2.
Zinc chloride | ZnCl2 or Cl2Zn | CID 5727 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.
Consider the example of zinc (Zn) shown here: Lewis dot diagram Bohr diagram. 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Complete the Lewis dot diagram and. Bohr diagram for Silicon (Si). Part B: Use the patterns in the periodic table to electron configurations for the following atoms. Symbol Group # Total # e- # valence e- (highest level) Electron.
The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1. Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F Lithium fluoride compound can be represented as Li + OR 1.
ZnS, Zinc Sulphide, Zinc Blend, Wurtzite In the zinc blend structure the sulphur ions form an fcc structure and the zinc ions occupy half of the tetrahedral sites in this structure to attain charge neutrality. The crystal has a lattice parameter of 0.541 nm.
In the first two columns, there is Group 1 and Group 2. The name goes as said, there is one dot around the elements in Group 1 and two around Group 2. In the Transition Metals, all of the groups (From Group 3-12) all have two dots around the elements. For Groups 13-18, Group 13 has 3 dots, Group 14 has 4, Group 15 has 5, and so on.
Q. In chemical compounds, covalent bonds form when. answer choices. the electronegativity difference between two atoms is very large. electrons are completely transferred between two metals. pairs of electrons are shared between two nonmetal atoms. two nonmetal atoms are attracted to each other by opposite charges.
(so u'll have four electrons (dots) surrounding zinc). this is the lewis structure and its that simple becoz there are no lone pairs on zinc as its valency is two. Wiki User ∙ 2012-04-23 05:14:17
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 –3 b. NO 3 – d. CO 3 2– 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
Answer (1 of 2): Instead of acting like an entitled egomaniac who thinks he's the smartest guy in the room like the other guy did, I'll provide an actual answer. Since nickel is a transition element, you have to manually write out its electron configuration and figure out how many electrons the l...




















:max_bytes(150000):strip_icc()/Scandium-58b6023e3df78cdcd83d49e1.jpg)




0 Response to "42 Lewis Dot Diagram For Zinc"
Post a Comment