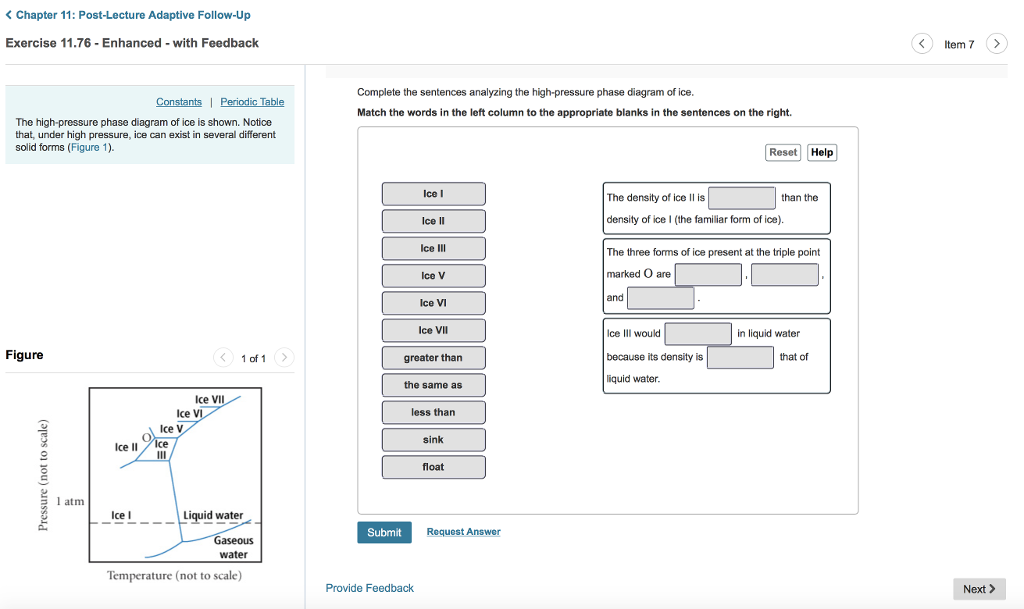
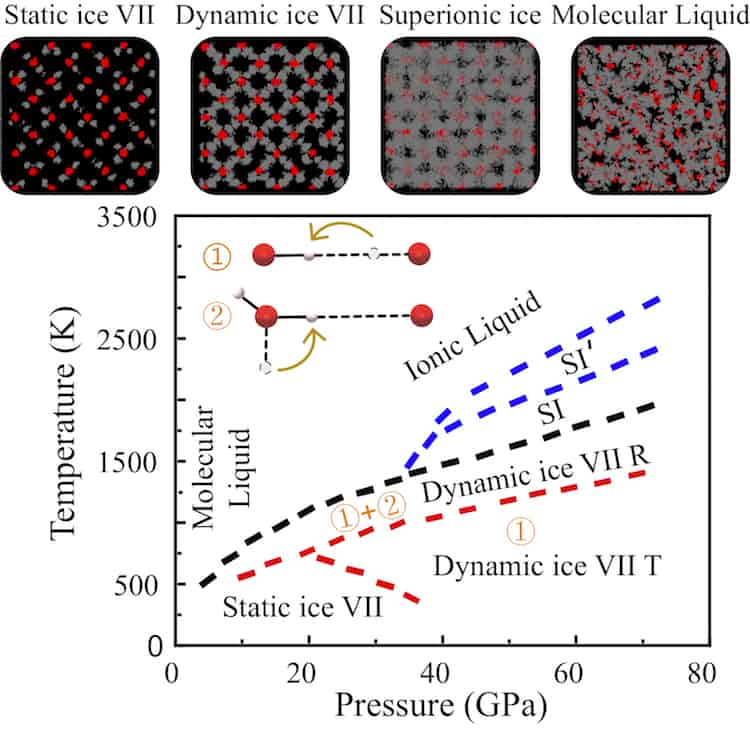
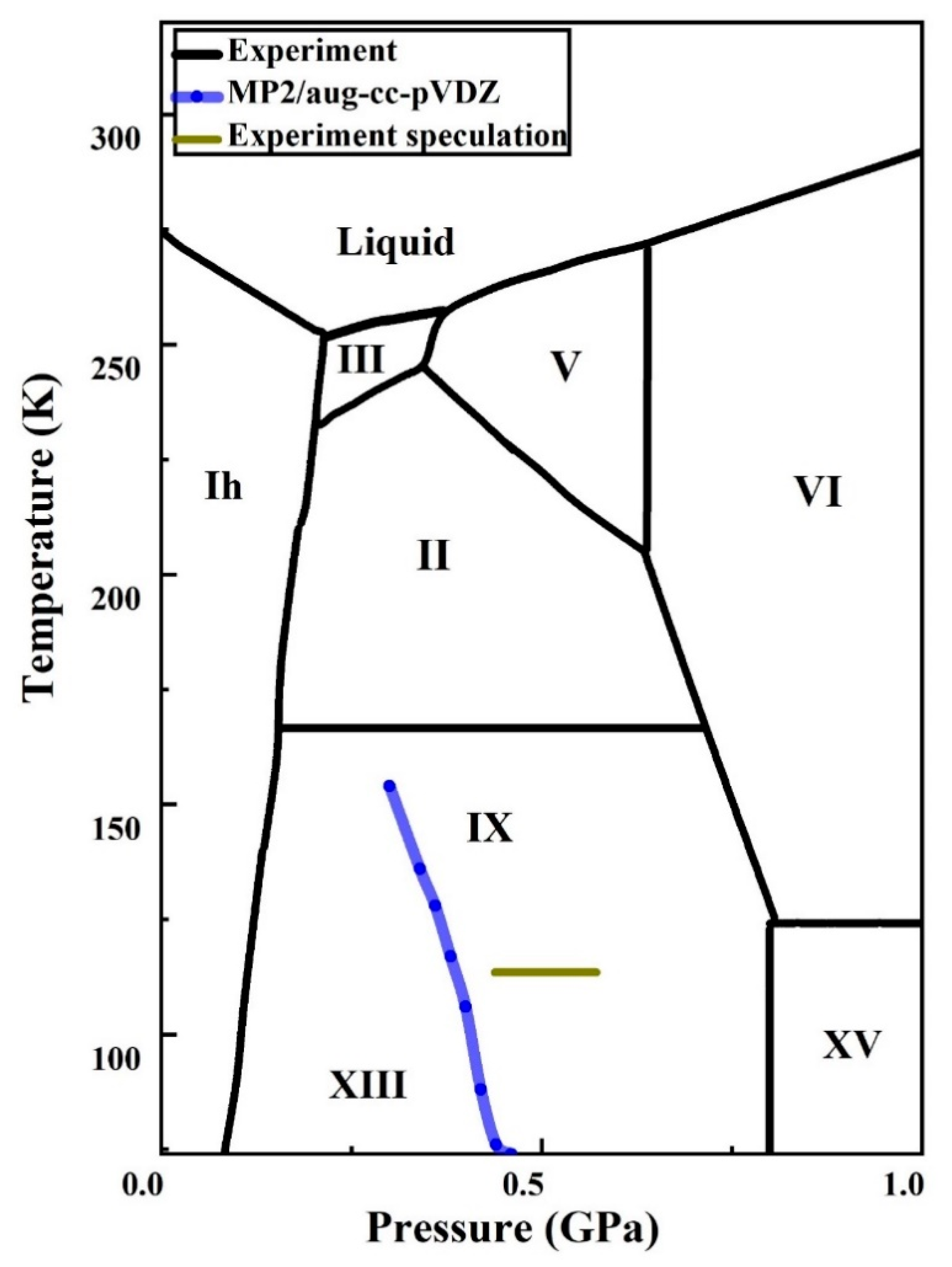
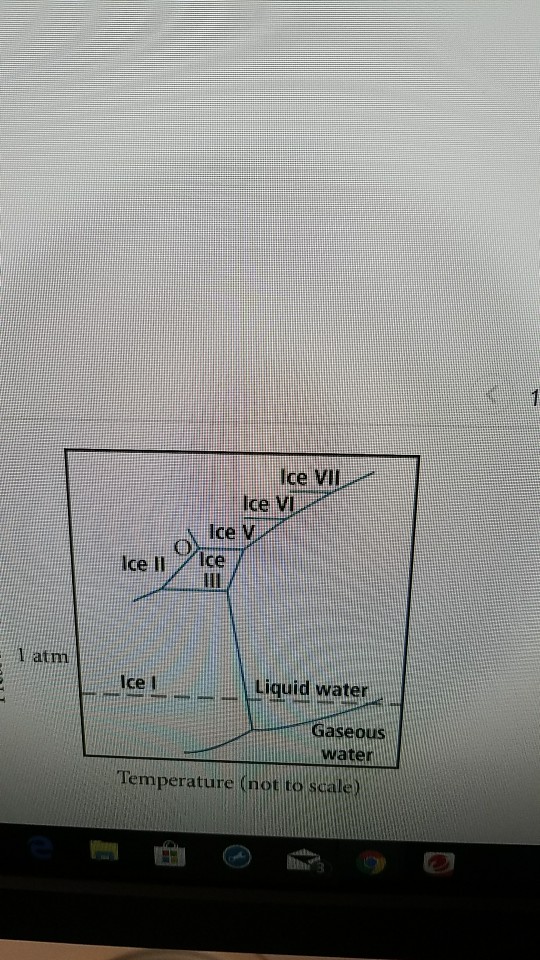
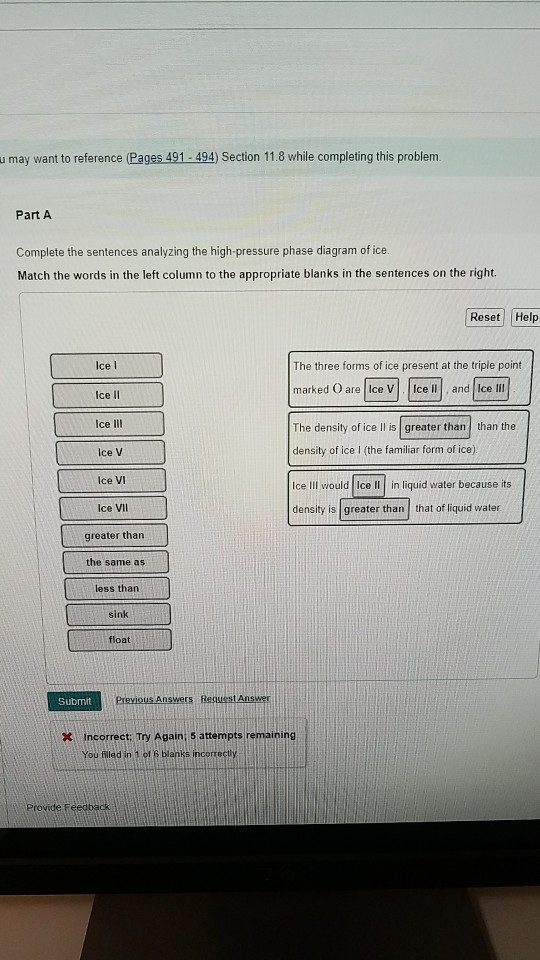
42 Complete The Sentences Analyzing The High-pressure Phase Diagram Of Ice.
When a diagram is complete, you should be able to place your pencil point at any place on the diagram, and predict what phase or phases are stable under those T-X conditions. Label each field with a label that describes the phase or phases that would be present if your experiment were conducted at the T and X conditions appropriate to that field. The critical point and the orange line in the ice-one phase space refer to the low-density (LDA) and high-density (HDA) forms of amorphous water (ice). Although generally accepted, the existence of this second, if metastable, critical point is impossible to prove at the present time and is disputed by some [ 200 , 618 , 628 ].
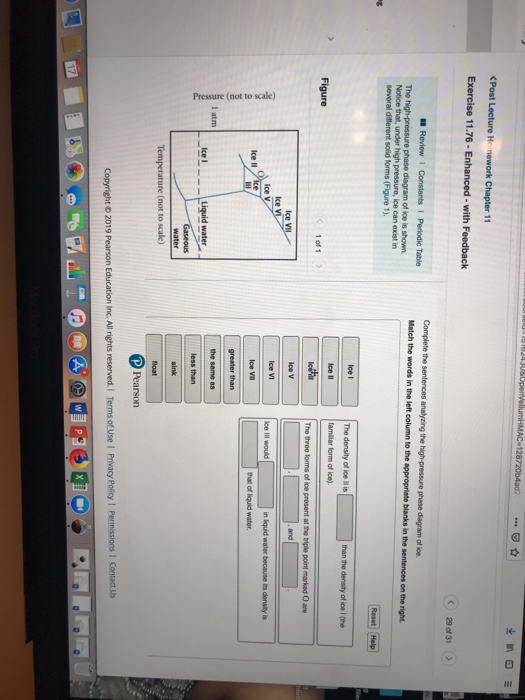
this's Jumper eleven Section Problems seventy six. The high pressure phase diagram of Isis shown here I've done the best I could to try to represent this out of a textbook note that under high pressure ice can exist in several different ice solid forms when three wish Three forms of ice are present at the triple point marked Oh, and it's The book points to where I've indicated rate.
Complete the sentences analyzing the high-pressure phase diagram of ice.
Question: u may want to reference (Pages 491-494) Section 11.8 while completing this problem Part A Complete the sentences analyzing the high-pressure phase diagram of ice. Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help Ice l Ice II ice!" Ice V Ice VI Ice VII The three forms of ice present. A dark color on water vapor imagery implies a lack of moisture in the mid and upper levels of the atmosphere. The surface, 850 mb, and 700 mb charts can be used to assess the low level moisture profile. The best way to analyze convective instability is by the use of a Skew-T diagram. Phase change is often shown in a diagram like the one below: Diagram of water phase changes When a substance is in a solid state, it can absorb a lot of energy in the form of heat until it hits.
Complete the sentences analyzing the high-pressure phase diagram of ice.. A truck has a minimum speed of 14 mph in high gear. When traveling x mph, the truck burns diesel fuel at the rate of 0.0086584(900/x +x) gal/mile Assuming that the truck can not be driven over 59 mph and that diesel fuel costs $1.21 a gallon, find the Several aspects of the phase diagram of water at high pressure are immensely controversial: the location of the melting line 5,10,11,12,13,14,15,16,17,18 and the existence, structure, physical. This chapter builds on the introduction to the arrangement of particles in materials that was covered in the chapter 'Solids, Liquids and Gases' of the Gr. 6 Matter and Materials curriculum. In Gr. 6, no distinction was made between atoms and molecules. These were grouped together and the generic term 'particle' was used to refer to these. Key Concepts. Since density is a characteristic property of a substance, each liquid has its own characteristic density. The density of a liquid determines whether it will float on or sink in another liquid. A liquid will float if it is less dense than the liquid it is placed in. A liquid will sink if it is more dense than the liquid it is.
The entropy of a substance is influenced by structure of the particles (atoms or molecules) that comprise the substance. With regard to atomic substances, heavier atoms possess greater entropy at a given temperature than lighter atoms, which is a consequence of the relation between a particle's mass and the spacing of quantized translational energy levels (which is a topic beyond the scope. Therefore, a pressure change has the opposite effect on those two phases. If ice is relatively near its melting point, it can be changed into liquid water by the application of pressure. The water molecules are actually closer together in the liquid phase than they are in the solid phase. Refer again to water’s phase diagram ( Figure above ). during the phase change. Intermolecular Forces. Intermolecular Forces • Calculate the enthalpy change upon converting 1.00 mol of ice at -25 °°°°C to water vapor (steam) at 125 °°°°C under a constant pressure of 1 atm. The specific heats of ice, water, and steam are 2.09 J/g-K, 4.18 J/g-K and 1.84 J/g-K, respectively. diagram the different phases of the cell cycle, labeling the parts of the cell that are pertinent. Labels may include the percentage of the time cells spend in each phase. summarize the following regarding meiosis: Meiosis occurs in sexual reproduction when a diploid cell produces four haploid daughter cells that can mature to become gametes.
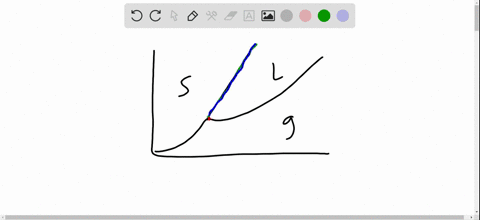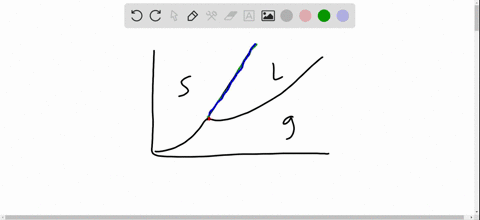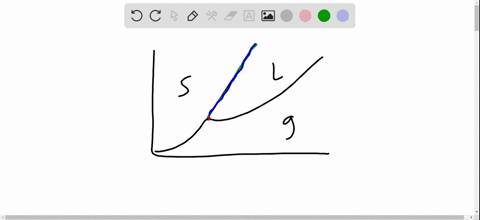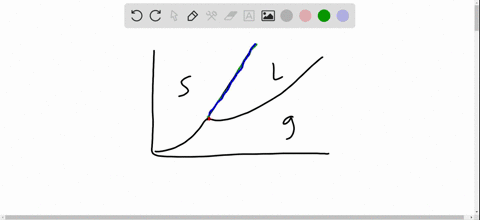
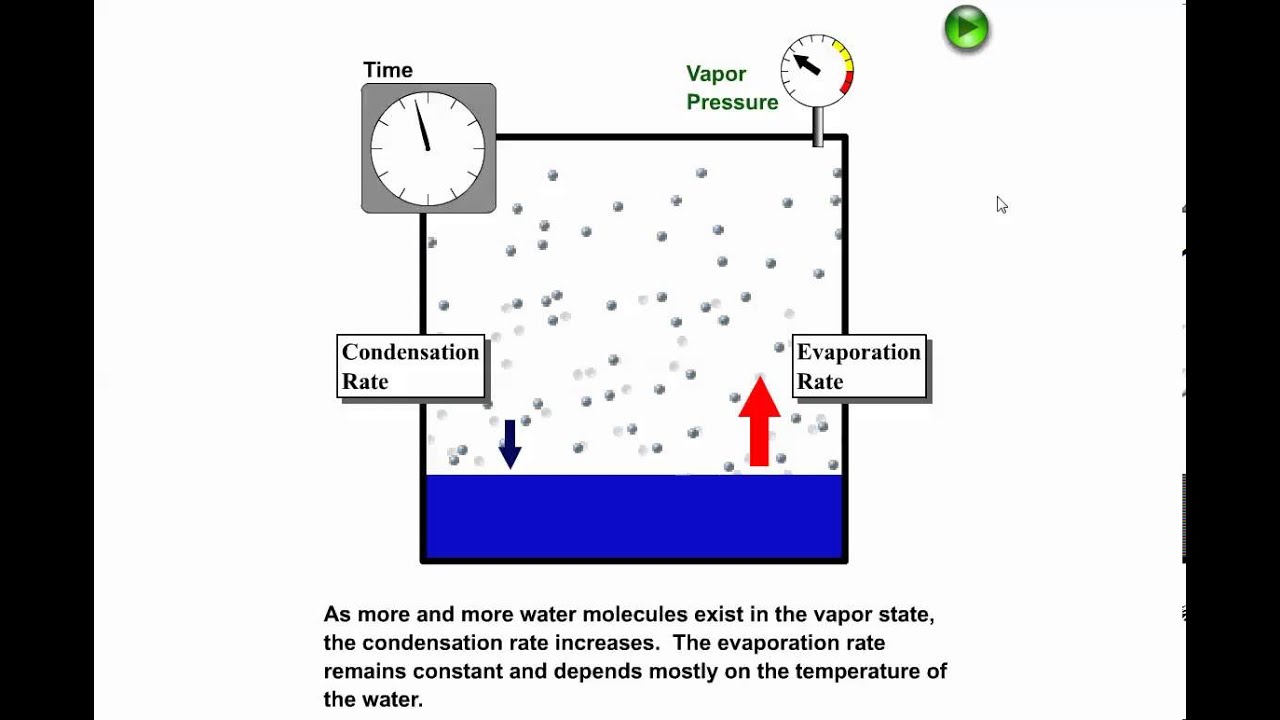
Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid. Fig. 4: the area under P‐V diagram represents the boundary work. Fig. 5: network done during a cycle. Polytropic Process During expansion and compression processes of real gases, pressure and volume are often related by PVn=C, where n and C are constants. The moving work for a polytropic Matter changes phases when it transforms from one state to another, such as from a liquid into a gas. Explore the definition, types, and examples of phase changes of matter. Understand how to. Phase change is often shown in a diagram like the one below: Diagram of water phase changes When a substance is in a solid state, it can absorb a lot of energy in the form of heat until it hits.
We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled “ice.”. Under these conditions, water exists only as a solid (ice).
Academia.edu is a platform for academics to share research papers.
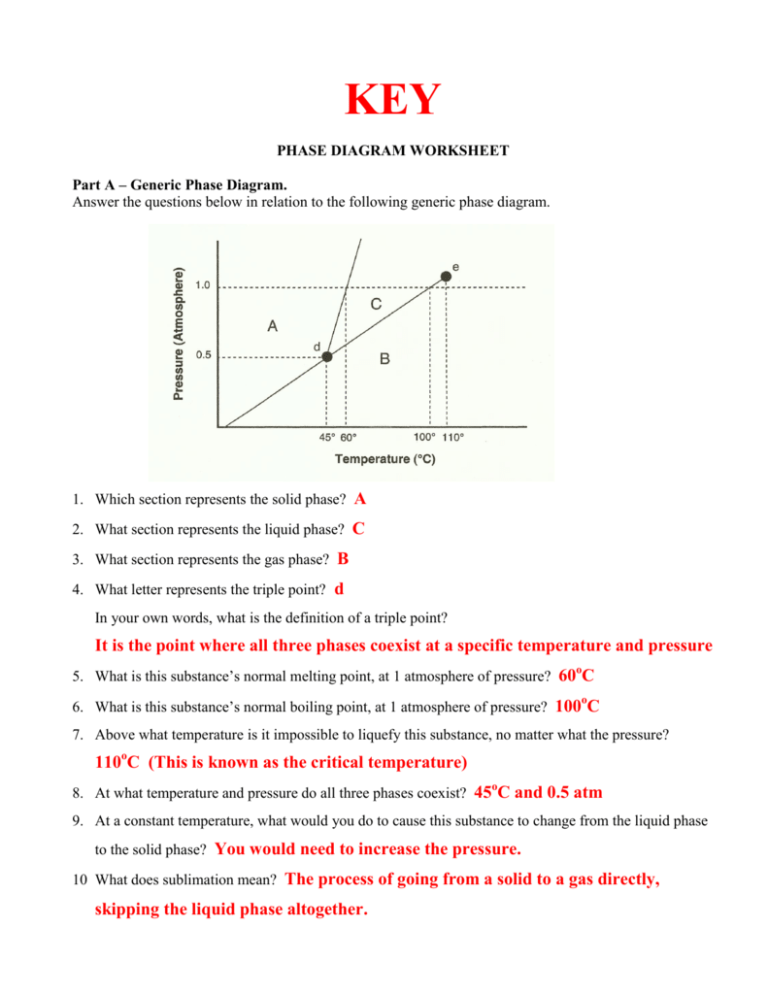
A phase diagram is a temperature-pressure plot that summarizes the conditions under which a substance exists as a solid, liquid, or gas. The curves that separate the phases are known as phase boundaries. Each phase boundary represents the equilibrium between the phases on either side of the curve. Identify the components of the phase diagram of.
ice cream. This results in a leftward shift of the supply curve for chocolate ice cream as ice-cream producers reduce the quantity of chocolate ice cream supplied at any given price. Ultimately, this leads to a rise in the equilibrium price and a fall in the equilibrium quantity. b.
To model the next phase, place your back to the sun, and hold the moon straight out in front of you (Earth) but still slightly overhead (see Diagram 2). Notice that it is this inclined orbit position that allows you to see the full half of the moon facing you lit up even when the Earth is between the sun and the moon — Earth's shadow does.
Brainly is the knowledge-sharing community where 350 million students and experts put their heads together to crack their toughest homework questions.
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Precipitation and the Water Cycle. Precipitation is water released from clouds in the form of rain, freezing rain, sleet, snow, or hail. It is the primary connection in the water cycle that provides for the delivery of atmospheric water to the Earth. Most precipitation falls as rain.
Brainly is the knowledge-sharing community where 350 million students and experts put their heads together to crack their toughest homework questions.
Question: u may want to reference (Pages 491-494) Section 11.8 while completing this problem Part A Complete the sentences analyzing the high-pressure phase diagram of ice. Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help Ice l Ice II ice!" Ice V Ice VI Ice VII The three forms of ice present.
She is also a Fellow and V ice-chair of the Design Research Society and Vice-chair of the Group for Learning in Art and Design. Adam Feather is a Consultant Geriatrician at Newham University Hospital Trust and a Senior Lecturer in Medical Education at Barts and The London, Queen Mary's School of Medicine and Dentistry.
The largest (and best) collection of online learning resources—guaranteed. Hundreds of expert tutors available 24/7. Get answers in as little as 15 minutes. Educators get free access to course content. Access syllabi, lecture content, assessments, and more from our network of college faculty. Neil Garg, Professor of Chemistry, University of.
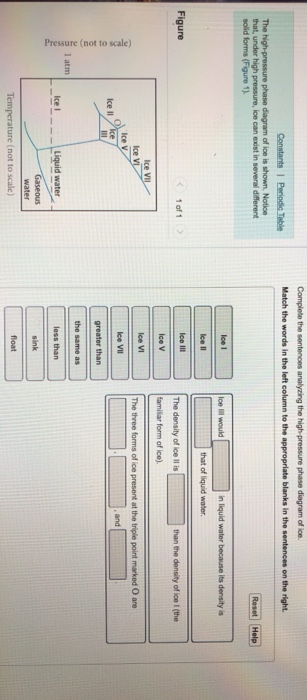
Transcribed image text: Constants 1 Periodic Table hat, under high pressure, ice can exist in several different solid forms (Figure 1). lce I n liquid water because its density is Figure 1 of 1 Ice III familiar form of ice). Ice lce VI lce VIl greater than Ice Ice The three forms of ice present at the triple point marked O are and Ice lI 1 atm water float Temperature (not to scale)
PROTOSTAR PHASE:-luminosity much greater than the Sun-radius much larger than the Sun-pressure and gravity are not precisely balanced-energy generated by gravitational contraction MAIN SEQUENCE PHASE:-energy generated by nuclear fusion-lasts about 10 billion years-surface radiates energy at same rate that core generates energy
7-26 During the isothermal heat addition process of a Carnot cycle, 900 kJ of heat is added to the working fluid from a source at 400°C. Determine (a) the entropy change of the working fluid, (b) the entropy change of the source, and (c) the total entropy change for the process.7-29 Refrigerant-134a enters the coils of the evaporator of a refrigeration
A dark color on water vapor imagery implies a lack of moisture in the mid and upper levels of the atmosphere. The surface, 850 mb, and 700 mb charts can be used to assess the low level moisture profile. The best way to analyze convective instability is by the use of a Skew-T diagram.
Consequently, they form liquids. Butane, C 4 H 10, is the fuel used in disposable lighters and is a gas at standard temperature and pressure. Inside the lighter's fuel compartment, the butane is compressed to a pressure that results in its condensation to the liquid state, as shown in Figure 3. Figure 3.
Sound waves traveling through a fluid such as air travel as longitudinal waves. Particles of the fluid (i.e., air) vibrate back and forth in the direction that the sound wave is moving. This back-and-forth longitudinal motion creates a pattern of compressions (high pressure regions) and rarefactions (low pressure regions). A detector of pressure at any location in the medium would detect.
Label the samples accordingly and complete the two sentences with labels that correctly describe seasonal sea ice melt and seawater freezing and the relationship to salinity.. water on the salty side of a semipermeable membrane is pushed under high pressure through the membrane to the freshwater side.... On the lower pie diagram shown below ...
It is at those plateaus that a phase change occurs. Heating Curve of Water The phase transitions of water. Analysis of a Heating Curve. Looking from left to right on the graph, there are five distinct parts to the heating curve: Solid ice is heated and the temperature increases until the normal freezing/melting point of zero degrees Celsius is.






















0 Response to "42 Complete The Sentences Analyzing The High-pressure Phase Diagram Of Ice."
Post a Comment