40 Titanium Electron Dot Diagram
Titanium tetrachloride is the inorganic compound with the formula TiCl 4.It is an important intermediate in the production of titanium metal and the pigment titanium dioxide.TiCl 4 is a volatile liquid. Upon contact with humid air, it forms spectacular opaque clouds of titanium dioxide (TiO 2) and hydrated hydrogen chloride.It is sometimes referred to as "tickle" or "tickle 4" due to the. Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na + ion, we note that the Na atom has a single valence electron in its Lewis diagram.
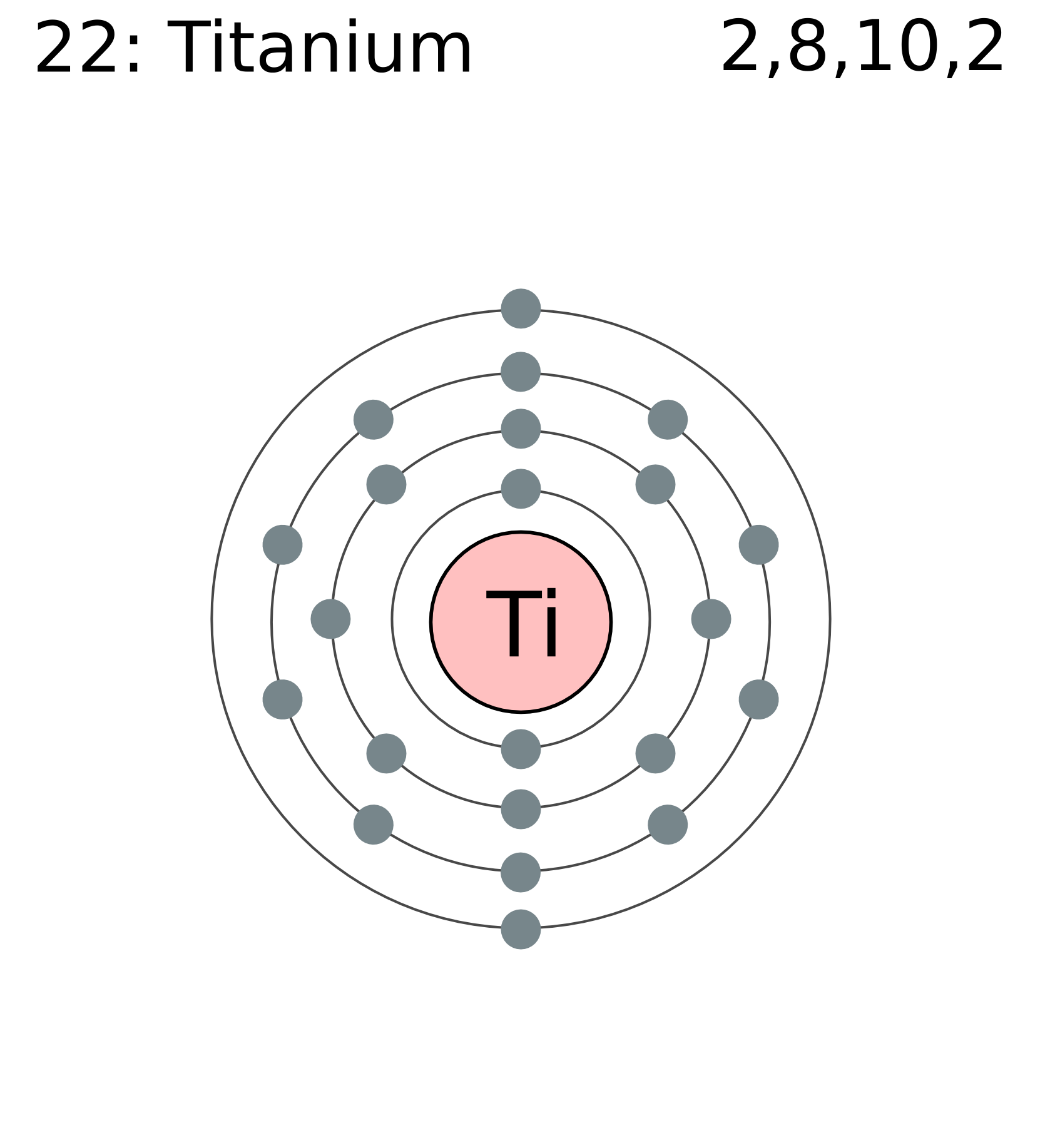
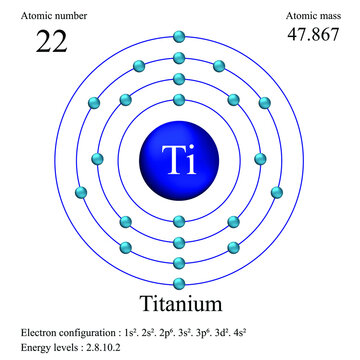
The structure of the titanium atom is complex, with 22 protons, 26 neutrons and 22 electrons. Creating a Bohr model of the atom is the best. Titanium has an atomic number of This means that it has 22 protons, and subsequently, as it is neutral, 22 electrons within its 'electron shells' (according to.
Titanium electron dot diagram
A Lewis structure is a structural representation of a molecule where dots are used to show electron positions around the atoms and lines or dot pairs represent covalent bonds between atoms. In an ionic bond, it's more like one atom donates an electron to the other atom. Nov 07, 2021 · ISSN 0021-9584. b. Sep 26, 2017 · Covalent Bond- Bond parameters (bond energy, bond length, bond angle)- Lewis dot structures. You should take formal charges into account with the Lewis structure for N3- to find the best structure for the molecule. Instead, the boron is electron deficient having only six electrons. 7) in the ANSWER BOOK. A step-by-step explanation of how to draw the Lewis dot structure for Mg (Magnesium). I show you where Magnesium is on the periodic table and how to determi...
Titanium electron dot diagram. Generally, pure titanium can crystallize in two crystal structures: α titanium and β titaniu. When it crystallizes at low temperatures (room temperature), the hexagonal close-packed (HCP) structure of alpha titanium is formed. While it crystallizes at high temperatures, the body-centered cubic (BCC) structure of beta titanium is formed. Electron domain geometry requires a 3-D diagram showing the tetrahedral arrangement. Question 10a- [1 Mark] Draw the Lewis (electron dot) structure for BrO − that obeys the octet rule. Sep 09, 2019 · a, Schematic structure of Ti 3 C 2 T x MXene. Surface terminations (T x) are a mixture of F, O, and OH. b, TEM image of Ti 3 C 2 T x MXene flakes. Scale bar, 500 nm. The corresponding selected. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are.
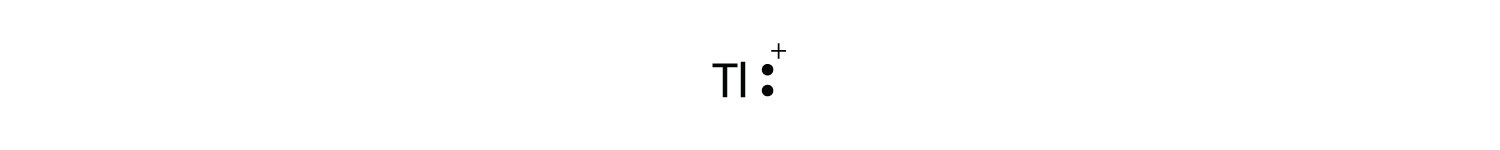
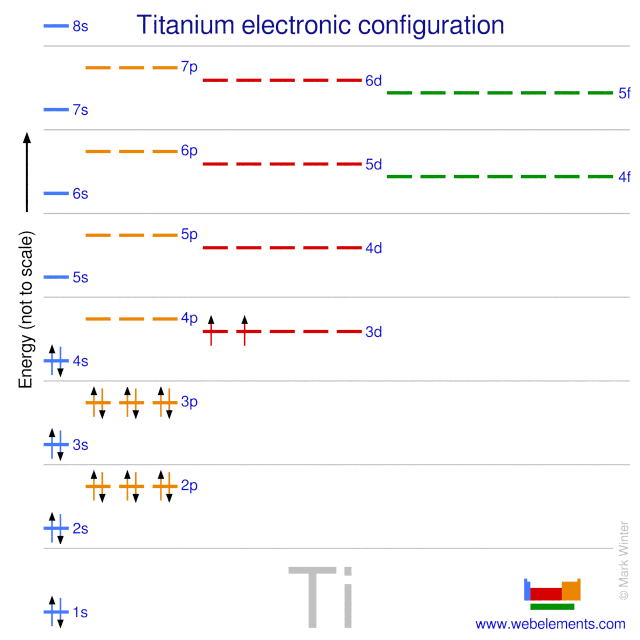
Write the electron configuration for the element titanium, {eq}\displaystyle \rm Ti {/eq}. Express the answer in order of increasing orbital energy. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the. Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. A step-by-step explanation of how to draw the Cs Lewis Dot Structure.For the Cs structure use the periodic table to find the total number of valence electron...
Nov 07, 2021 · ISSN 0021-9584. b. Sep 26, 2017 · Covalent Bond- Bond parameters (bond energy, bond length, bond angle)- Lewis dot structures. You should take formal charges into account with the Lewis structure for N3- to find the best structure for the molecule. Instead, the boron is electron deficient having only six electrons. 7) in the ANSWER BOOK. A Lewis structure is a structural representation of a molecule where dots are used to show electron positions around the atoms and lines or dot pairs represent covalent bonds between atoms. In an ionic bond, it's more like one atom donates an electron to the other atom. The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. But these electrons are concentrated in three places: The two C-O single bonds and the C=O double bond. Repulsions between these electrons are minimized when the three oxygen atoms are arranged toward the corners of an. A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, the beams of which are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms (oscilloscope), pictures (television set, computer monitor), radar targets, or other phenomena.
This element exists in the solid state at room temperature and at normal atmospheric pressure and is found in emerald gemstones. It is known to be one of the following elements: carbon, germanium, sulfur, cesium, beryllium, or argon. Identify the element based on the electron-dot structure. a) Si 1s²2s²2p⁶3s²3p².
Lewis Dot Diagrams of Selected Elements. Lewis Symbols: Electron Configuration into Shells: Index Chemical concepts Chemistry of the Elements Periodic Table. HyperPhysics***** Quantum Physics : R Nave: Go Back: Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its.
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells. These energy levels contain sub-shells, or orbitals, each of.
Lewis structure, electron dot diagram, electron dot structure... Why is titanium dot diagram 3? Titanium has 3 valence electrons. Thus it requires three dots per the rules for drawing a dot diagram.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, The electron dot diagram for helium, with two valence electrons, is as follows: Helium. By putting the two. dot diagram for each element. a) titanium.Nov 16, · Titanium has an atomic number of 22 i.e. 22 electrons in its neutral state.
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
Nov 07, 2021 · Photocatalytic degradation of MB was carried out over Au/TiO 2 upon visible irradiation to identify the contribution of H 2 on reactivity of photogenerated hot holes. A Xe lamp coupled with 495 nm cutoff-filter was utilized to rule out the contribution from TiO 2 (absorbance edge at ~400 nm) and oxygen was depleted by He or H 2 before the reaction to exclude possible electron-mediated.
The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
The Lewis Electron-Dot Symbols of Elements. Gilbert N Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen the Bohr's diagram for the first 18 elements. Sometimes it is more convenient to represent the elements by its Lewis electron dot symbol.
Delocalization effects can also be understood using molecular orbital theory as a lens, more specifically, by examining the intramolecular interaction of the unpaired electron with a donating group’s pair of electrons or the empty π* orbital of an electron-withdrawing group in the form of a molecular orbital diagram. The HOMO of a radical is.
Explanation: Titanium lies in Group 4 of the Periodic Table and has 4 valence electrons. But we would seldom write a Lewis dot structure for titanium metal. T iCl4 is a common titanium compound (and is used for skywriting by light aircraft, why?), but so is solid T iCl3; T iCl2 is also known. Answer link.
Titanium acetate | C8H16O8Ti | CID 59044991 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.
3. Draw the Lewis electron dot diagram for each element. a) strontium b) silicon. 4. Draw the Lewis electron dot diagram for each element. a) titanium b) phosphorus. 5. Draw the Lewis electron dot diagram for each ion. a) Mg 2+ b) S 2−. 6. Draw the Lewis electron dot diagram for each ion. a) Fe 2+ b) N 3−
Ti Titanium Element information, facts. Titanium properties, uses and trends | Periodic Table of the Elements - complete information about the titanium element - Facts, atomic mass, melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron configuration, Chemical Properties titanium.
A step-by-step explanation of how to draw the Lewis dot structure for Mg (Magnesium). I show you where Magnesium is on the periodic table and how to determi...
Dec 04, 2019 · The red dot is the centre of the previous grain, which has grown to the size of the circle.. dashed line) based on the titanium–copper equilibrium phase diagram. However, no cracks in the as...
For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
Skip to page content; Skip to site menu on this page. Periodic Table of Elements Element Titanium - Ti. Comprehensive data on the chemical element Titanium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Titanium.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.
Quantum dots. (A) Emission of different colors of light while exciting UV light on quantum dot solutions. (B) Zinc oxide, carboxyl groups, and oligonucleotides coating on the surface of the quantum dot to facilitate DNA binding. Adapted from Wikipedia, 2017. DNA-functionalization of quantum dots. [Online]. [5 January 2018].
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4 s 2 3 d 6 ) is as follows: Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
Titanium dioxide is a titanium oxide with the formula TiO2. A naturally occurring oxide sourced from ilmenite, rutile and anatase, it has a wide range of applications. It has a role as a food colouring. Titanium dioxide, also known as titanium (IV) oxide or titania, is the naturally occurring oxide of titanium.
:max_bytes(150000):strip_icc()/Titanium-58b602395f9b5860464c4d8e.jpg)

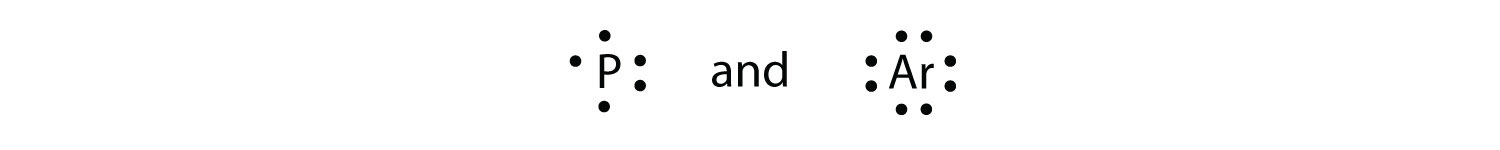








:max_bytes(150000):strip_icc()/Scandium-58b6023e3df78cdcd83d49e1.jpg)
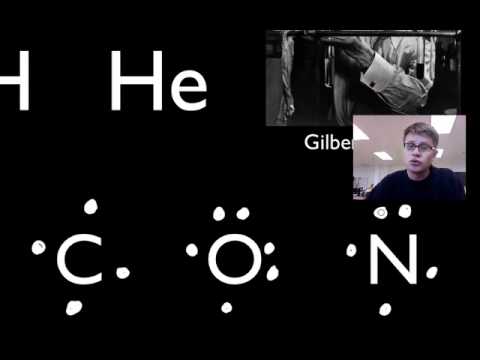










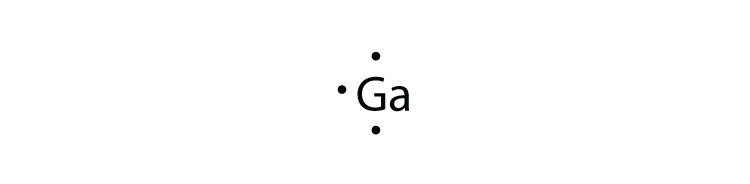



0 Response to "40 Titanium Electron Dot Diagram"
Post a Comment