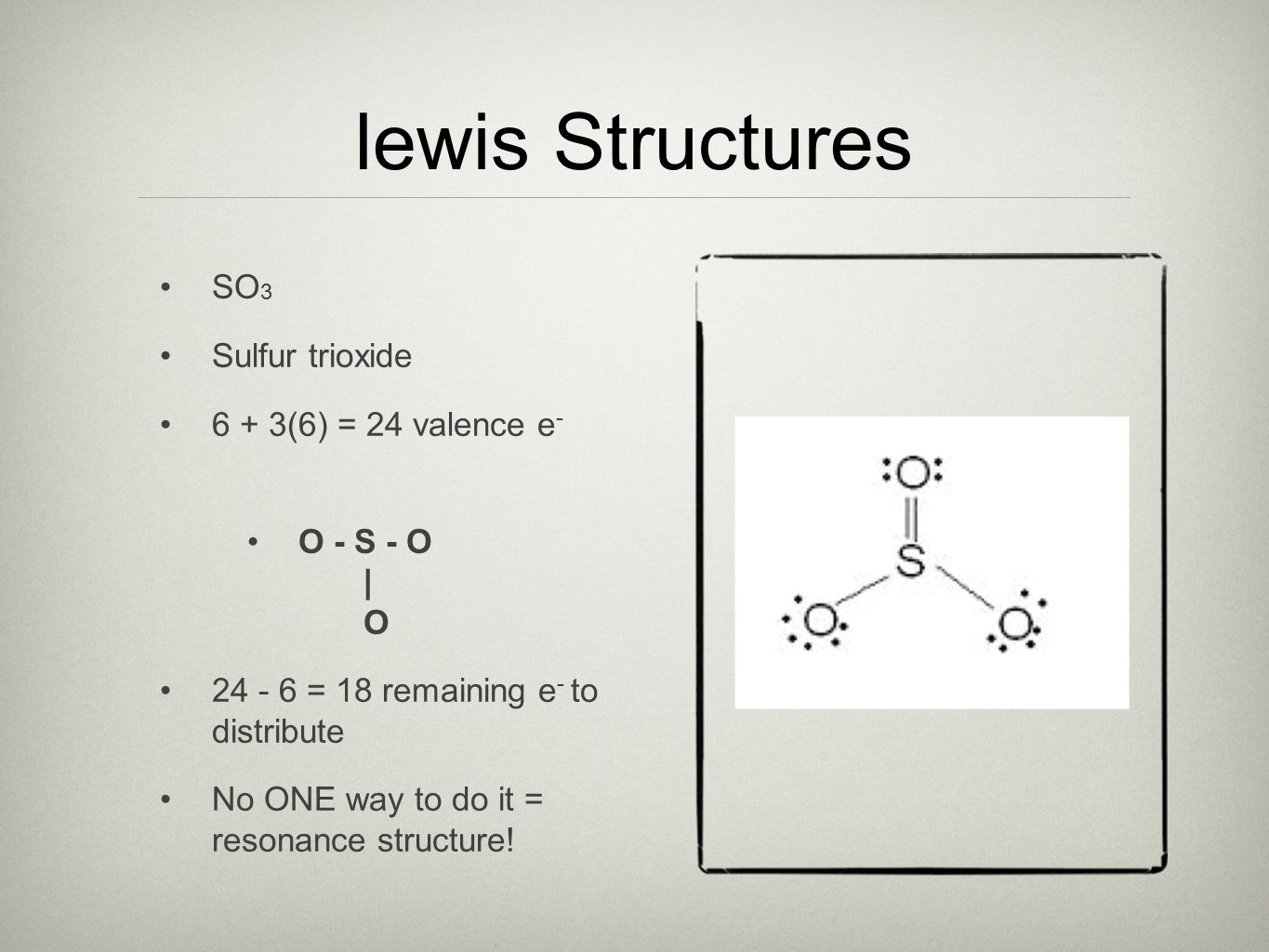
38 Lewis Dot Diagram For So3
SBr2 Lewis structure diagram in this phase. The electronegativity value in periodic groups grows from left to right in the periodic table and drops from top to bottom. The first step is to put six valence electrons around the sulfur atom as given in the figure. Step-2: Lewis Structure of Let's do the SO3 2- Lewis structure. For the SO3 2- compound, we have 26 total valence electrons, and that includes these two electrons up here--there are two extra valence electrons. So we have 26. Let's put the Sulfur at the center and the Oxygens around the outside. Put two electrons between the atoms to form chemical bonds. We've used 6.
The Lewis structures of Nitrogen Monoxide NO are drawn. 1.S. 2.Total valence electron is 6+3x6 - (-2)= 26. 3.Connect the atoms with single bonds. 4.P=6n+2 -V =6x4 +2 -26 = 26-26 = 0 ; 0 electron which must be shared through pi bonding and unshared i.e; Therefore, there is 0 double bond and an electron.. 5.Distribution of other electron.
Lewis dot diagram for so3
Lewis structures or electron dot structures - SO3 Lewis structure 1. Simple Procedure for writing Lewis Structures - Example: SO3 FOR CLICKABLE LINKS AND A DETAILED DESCRIPTION OF THE SIMPLE METHOD DESCRIBED BELOW PLEASE SEE PAGE 4 OF THIS PRESENTATION A simple procedure for writing Lewis structures is given in a previous article entitled "Lewis Structures and the Octet Rule". Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3. Draw The Lewis Dot Structure For So3 Slidesharedocs. So3 Drawing And Model On Vimeo. Write The Resonance Structure For So3 No2 And No3. So3 2 Molecular Geometry Shape And Bond Angles Youtube. Hybridization Of So3 Sulphur Trioxide Hybridization Of.
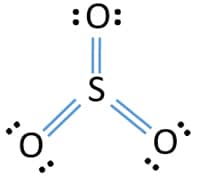
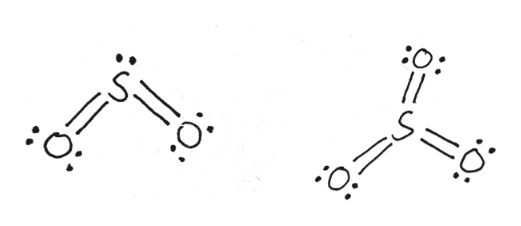
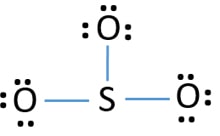
Lewis dot diagram for so3. In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. Step-3: Lewis dot Structure for SO3 generated from step-1 and. There are seven resonance structures for "SO"_3. > When you draw the Lewis structure, you first get the three structures at the top. In each of them, "S" has a formal charge of +2 and two of the "O" atoms have formal charges of -1. In each of the three structures in the middle, "S" has a formal charge of +1 and one of the "O" atoms has a formal charge of -1. In the bottom structure, all atoms. Draw The Lewis Dot Structure For So3 Slidesharedocs. So3 Drawing And Model On Vimeo. Write The Resonance Structure For So3 No2 And No3. So3 2 Molecular Geometry Shape And Bond Angles Youtube. Hybridization Of So3 Sulphur Trioxide Hybridization Of. in the previous video we looked at the dot structure for sulphur dioxide 9 through out two resonance structures so the resonance structure on the left and the resonance structure on the right and some people disagreed with me and said that's not the dot structure for sulphur dioxide the dot structure for sulphur dioxide has sulphur with a double bond to an oxygen on the left and two lone pairs.
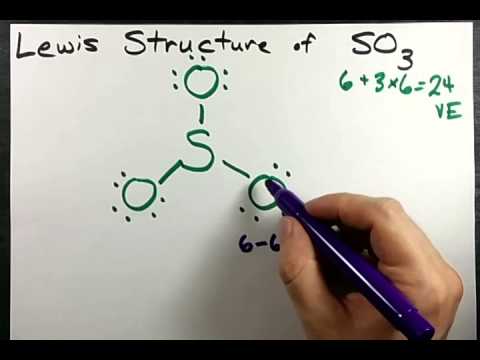
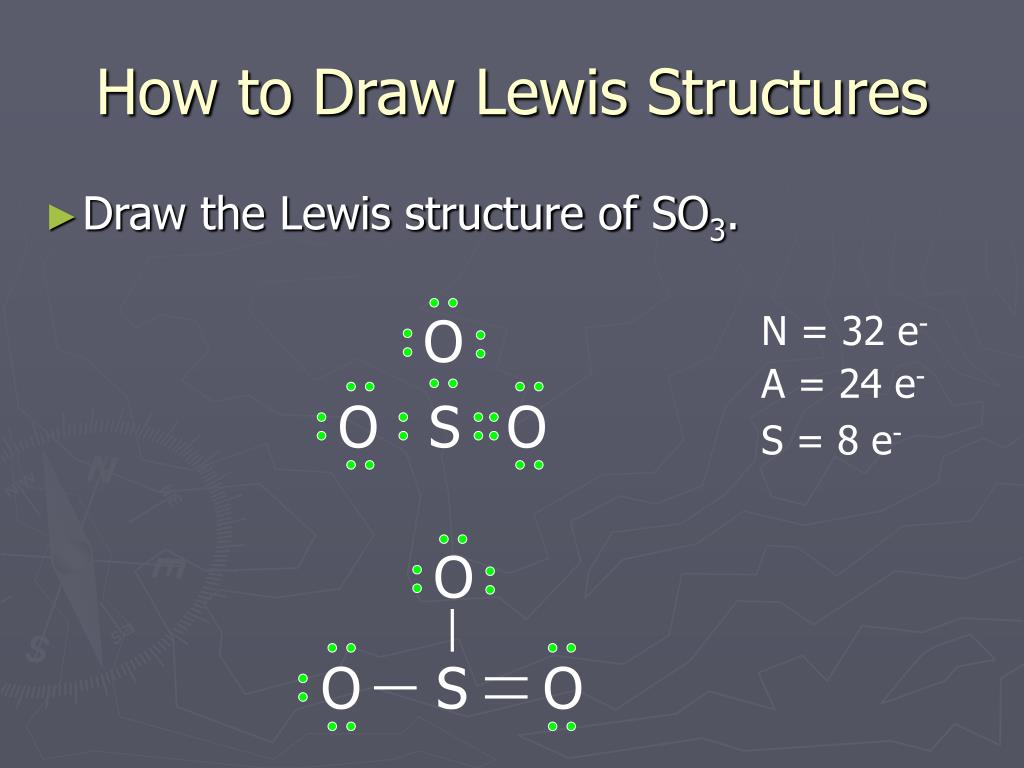
CO 2. BC13 3. SO3 4. CH4. Question: Lewis dot structure Molecular Formula Total valence Electrons Electron Domains (Basis of VSEPR) Molecular Geometry (shape) or Polar (P) Hybridization of central Nonpolar atom (NP) 1. CO 2. So 6 minus zero minus 12 over 2; so 6 minus 6 equals zero. So we can write the formal charge for Sulfur as zero. So we have formal charges of zero for each of the atoms in SO3. That makes this the best Lewis structure for SO3. This is Dr. B., and thanks for watching. 2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii. A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number...
Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen. Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3. This problem has been solved! Write a single Lewis structure for SO3. Draw the Lewis dot structure for SO3. Include all lone pairs of electrons. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Website-http://www.kentchemistry /links/bonding/LewisDotTutorials/SO3.htmI quickly take you through how to draw the Lewis Structure of SO3 (Sulfur Trioxid...
Lewis structures or electron dot structures - SO3 Lewis structure 1. Simple Procedure for writing Lewis Structures - Example: SO3 FOR CLICKABLE LINKS AND A DETAILED DESCRIPTION OF THE SIMPLE METHOD DESCRIBED BELOW PLEASE SEE PAGE 4 OF THIS PRESENTATION A simple procedure for writing Lewis structures is given in a previous article entitled "Lewis Structures and the Octet Rule".
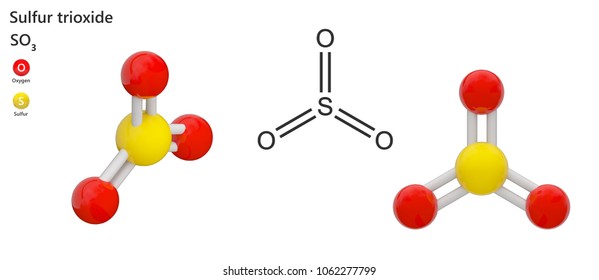
SO3 Molecular Geometry, Lewis Structure, and Polarity Explained. SO3 stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes.
How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check.
Dot and cross diagram of so2 and so3 · Structure and bonding Well it's hard to draw in a dot cross because of the resonance, but a google. The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms.
simple procedure for drawing the SO3 lewis structure, so3 lewis electron dot structure, electrostatic potential, ESP, resonance structures of so3, Lewis structures of SO3| Lewis configuration of sulfur trioxide SO3, octet rule, SO3 Lewis structure, π bonds, 2π electrons, multiple bonds, stable resonance, resonance structures, so3 resonance or double bonds, lewis structures and the octet.
Answer: Please read these instructions, if they do not make sense please PM me and we can set up an online session so I can facetime with you and get you squared away. Alright, here we go! Let's start off by differentiating between SO3 and SO3 2- SO3 is Sulfur Trioxide SO3²¯ is Sulfite They b...
Nov 11, 2021 · H2co resonance structures
C2f2 lewis structure molecular geometry. Contact. C2f2 lewis structure molecular geometry Services. Write for us. For Recruiters. Books. Resources.
Nov 13, 2021 · The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. The Lewis structure of sulfur trioxide (SO3) molecule is drawn by: First, look for the total number of valence electrons in a single sulfur trioxide (SO3) molecule, which is twenty-four.
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
What is the Lewis dot structure of SO3? It is six for a single sulfur trioxide (SO3) molecule, where both sulfur and each oxygen atom need two valence electrons to stabilize their atom. Next, look for the number and type of bonds forming within a single sulfur trioxide (SO3) molecule.
Lets do the so3 2 lewis structure. Lewis dot diagram for so3. So 6 minus 6 equals zero. 3 oxygens branch out. Alright here we go. So we have 26. For the so3 2 compound we have 26 total valence electrons and that includes these two electrons up here there are two extra valence electrons. I quickly take you through how to draw the lewis structure.
Seo3 shape. Seo3 shape
Lewis dot structures, as you have learned, are a way to diagram an element and easily show its valence electrons. A Lewis dot structure is a diagram that shows the valence electrons in an element.
Automatic procedure to construct Lewis dot structures, so32-, pi an d - Sulfite Ion SO3-2 Lewis structure, draw the lewis structure for the sulfite ion, Dot structure SO3-, so3 2-, SO32-,Sulfite,Ion,Lewis,dot,structure,hybridization,chemistry,bond,angle,drawing, ib, ap, a, SO3 2- Lewis Structure,Lewis Structure for SO3 2-,Lewis Structure,SO3 2-,SO3 2- Electron Dot Structure,Electron Dot.
The LibreTexts libraries are Powered by MindTouch ® and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
Sulfur Trioxide (SO 3) Lewis Structure, Hybridization | Drawing Steps. Sulfur trioxide molecule contains one sulfur atom and three oxygen atoms. We will construct the lewis structure of SO 3 molecule by following VSEPR theory rules and considering stability of intermediate structures. Finally, after obtaining the lewis structure of SO 3, we can determine the hybridization of atoms.
Lewis Diagram For Seo3. A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3). The structure and arrows are given, simply add the remaining bonds and lone. (1.
The central atom of this molecule is carbon. Simple molecules ch 4 nh 3 h 2o sif 4 ncl 3 group b. Draw the Lewis Dot structures for H 2, HF, LiH, O 2 and NO. Your Lewis structures of atoms can be used to build molecules. = 4/3. 7% carbon and 14. The Lewis electron dot structures of a few molecules are illustrated in this subsection.
Sio2 lewis structure molecular geometry. 5a sbr2 datasheet, inventory. 2 lone pairs: bent: 4. Silicon Dioxide, Silica | Chemistry, Class 11, p-Block Sio2 lewis structure molecular geometry Jan 29, 2021 · Sulfur Dioxide Lewis Structure Molecule Molecular Geometry There are four valance electrons in silicon therefore there will be 4 dots in your electron dot diagram.
4.7/5 (37 Views. 34 Votes) Drawing the Lewis Structure for SO3 (Sulfur Trioxide) It is a form of pollution. SO3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO3. Be sure to check the formal charges for the Lewis structure for SO3. Complete answer to this is here.
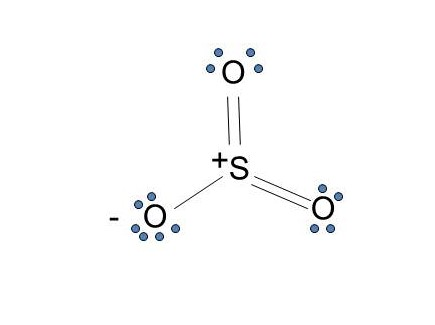
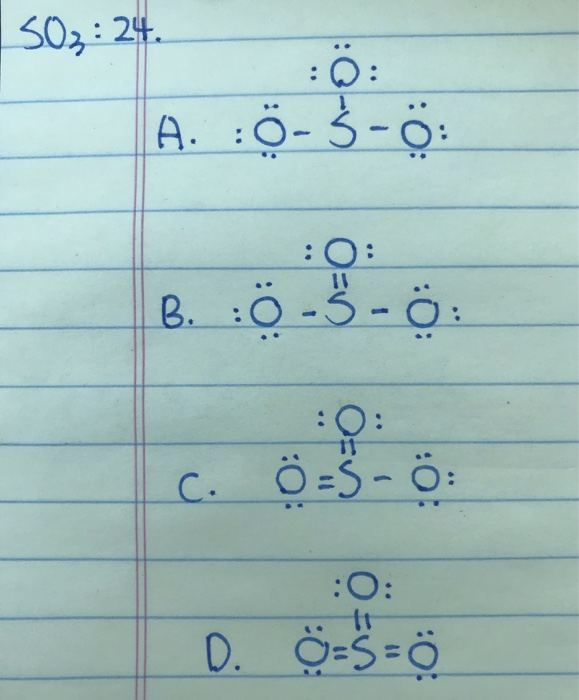
Answer (1 of 2): SO3 does not have the extra 2 electrons (SO3) 2- has. Therefore, the lewis dot structures will actually be very different. As shown below, The lewis structure of SO3 is being considered when no electrons will interfere with the synthesis of SO3, however this will change dramatic...
Smart Balbol on this occasion will inform draw the lewis dot structure of so3 for readers who need the content. Dear friends who really want to know draw the lewis dot structure of so3 can read the article En to the end so that the content written today is clearer October 27, 2021. The author who has the nickname Ali Maksum is a verified author on the Balbol website and of course related.
How many bonds should be drawn in the Lewis-dot structure for SO3? apex users its defenatley 3. Is SO3 a Lewis base or Lewis acid? I think it is acid, because there is a question that asks the.
Nov 17, 2021 · The Lewis dot structure shows the unpaired electrons or the lone pairs in the end.. An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory.... Previous Article SO3 Lewis Structure, Molecular Geometry, and Hybridization. Next ...
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.


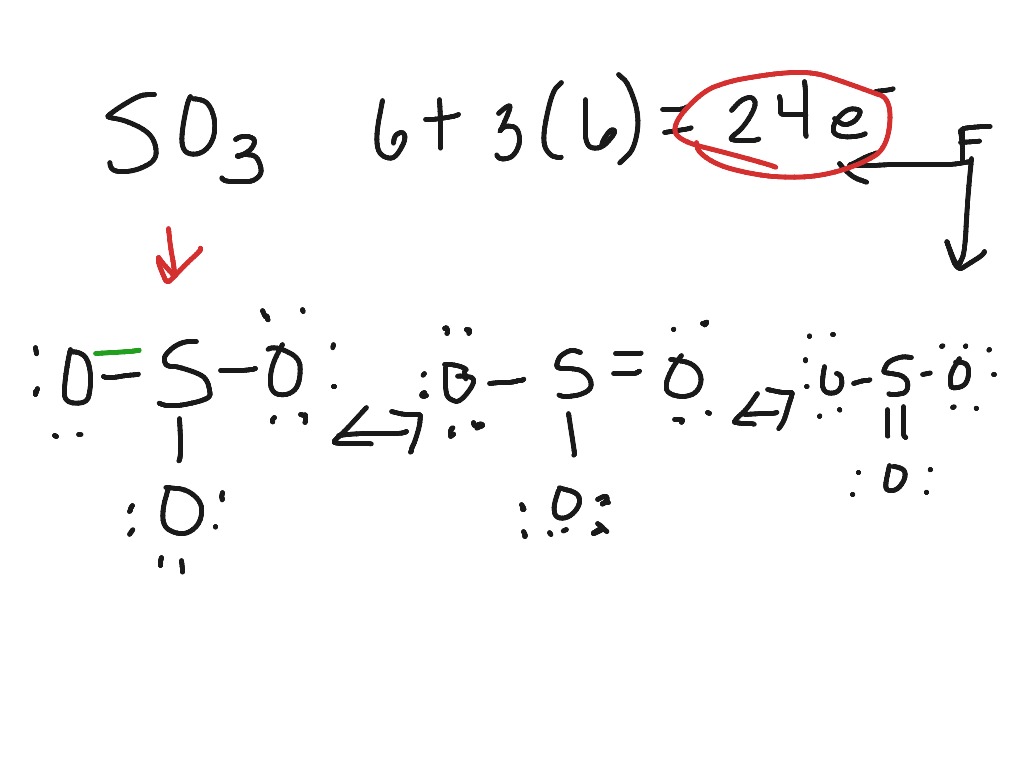






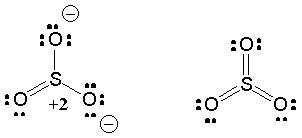










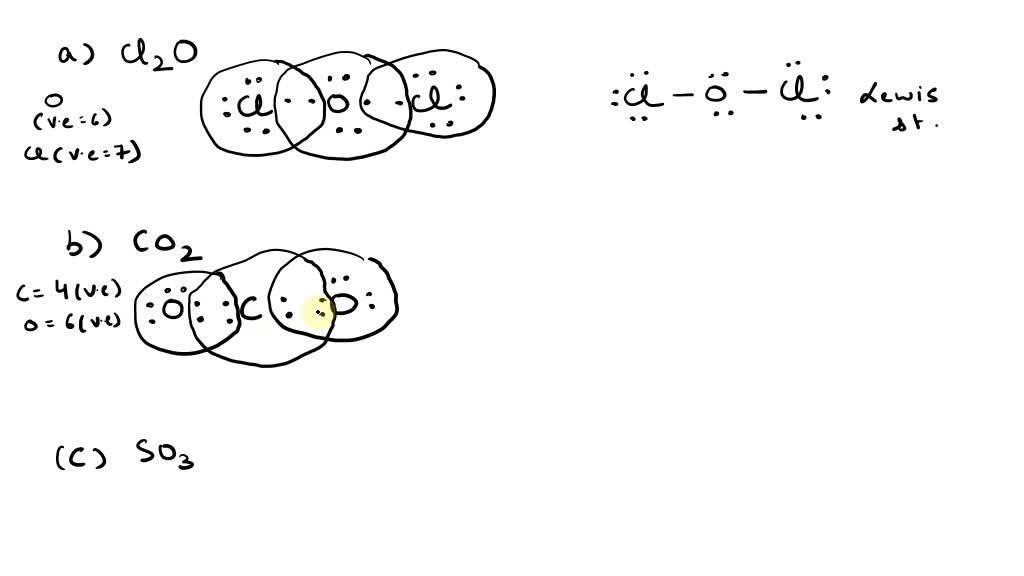
0 Response to "38 Lewis Dot Diagram For So3"
Post a Comment