37 Molecular Orbital Diagram Of H2
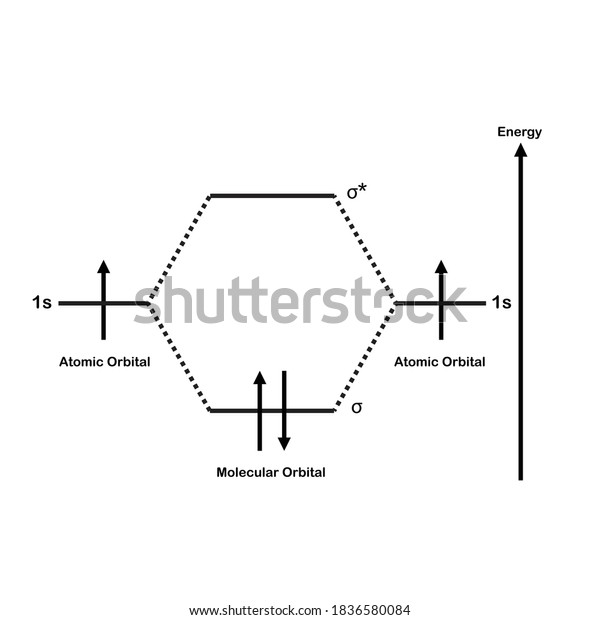
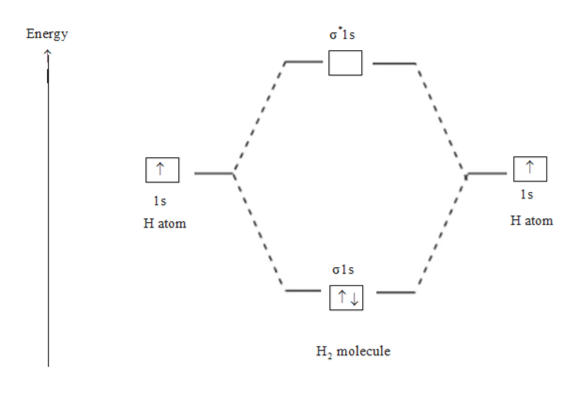
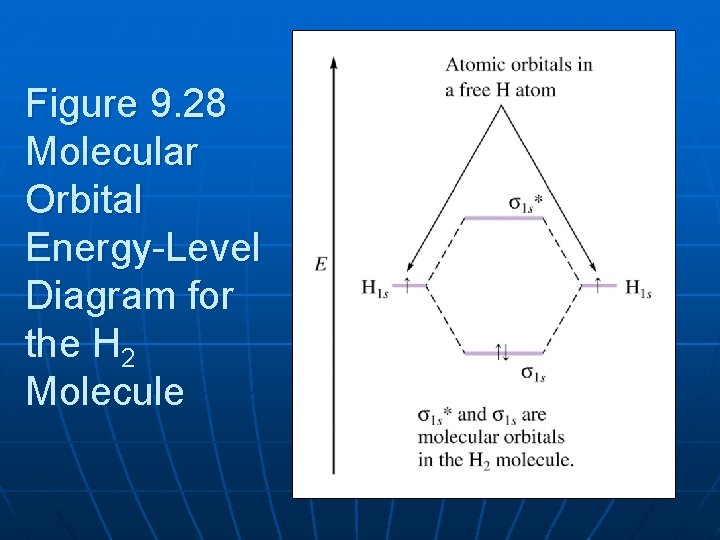
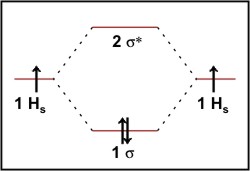
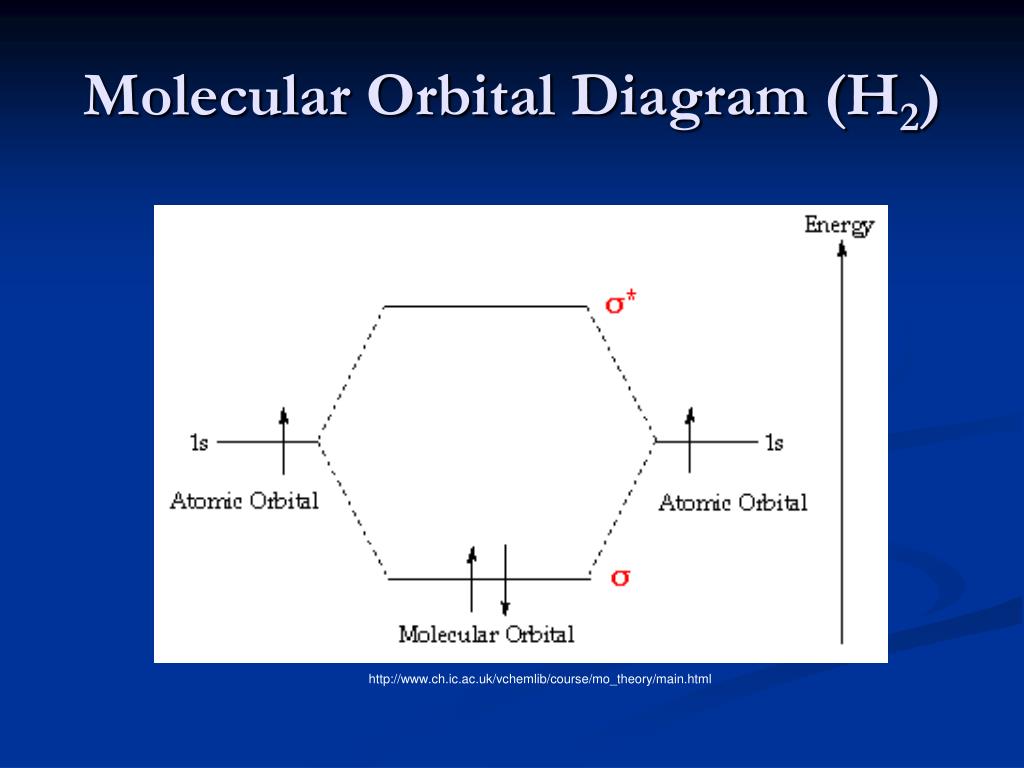
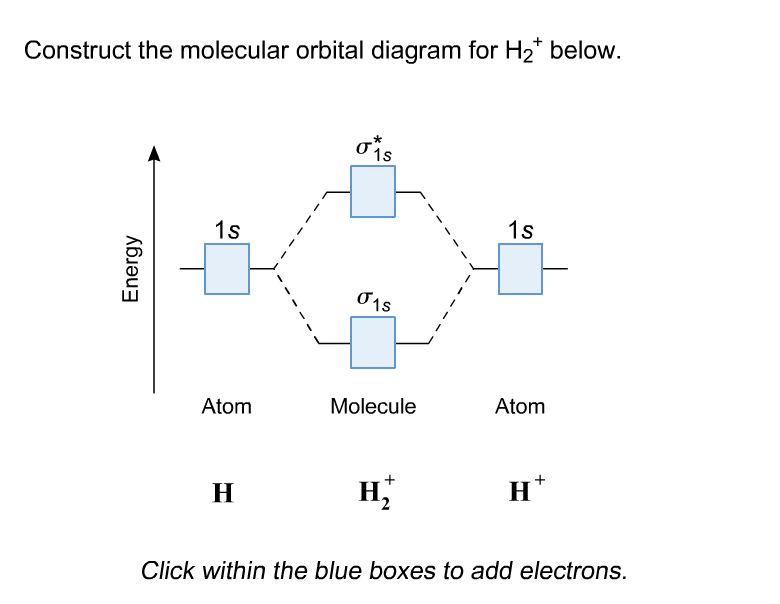
Hydrogen Molecular Orbital Diagram Atomic hydrogen has 1 electron in a 1s orbital. Of course, there are 2s, 2p, 3s, 3p, etc. empty orbitals at higher energy. Let's just consider the 1s orbitals. Remember that an orbital is a mathematical function that describes the probability of finding an electron in space. Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Each boron atom has one 2s and three 2p valence orbitals. In order to predict the bond order molecular orbital diagram for h2 is to be drawn. Molecular orbitals of h 2 the molecular orbital approach is one explanation for the ceh h bond.

Molecular orbital diagram of h2
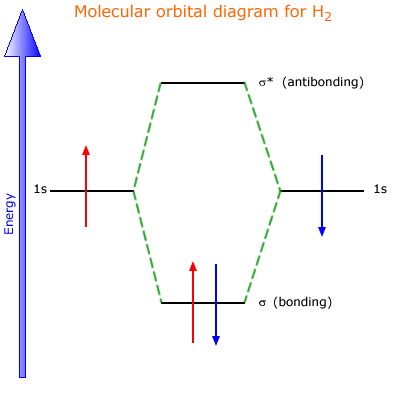
Molecular orbital diagram of h2. When creating the molecular orbitals from the p orbitals notice the three atomic orbitals split into three molecular orbitals a singly degenerate σ and a doubly degenerate π orbital. Molecular orbitals of h2 and he2. Bonding mos antibonding mos and bond order. Evaluate the ground state electronic energy based. 1. Problem: Draw MO energy diagram s for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. How to write simple Molecular Orbital Diagram s and. Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me.
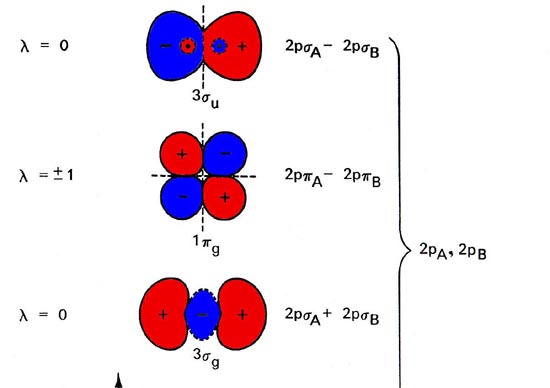
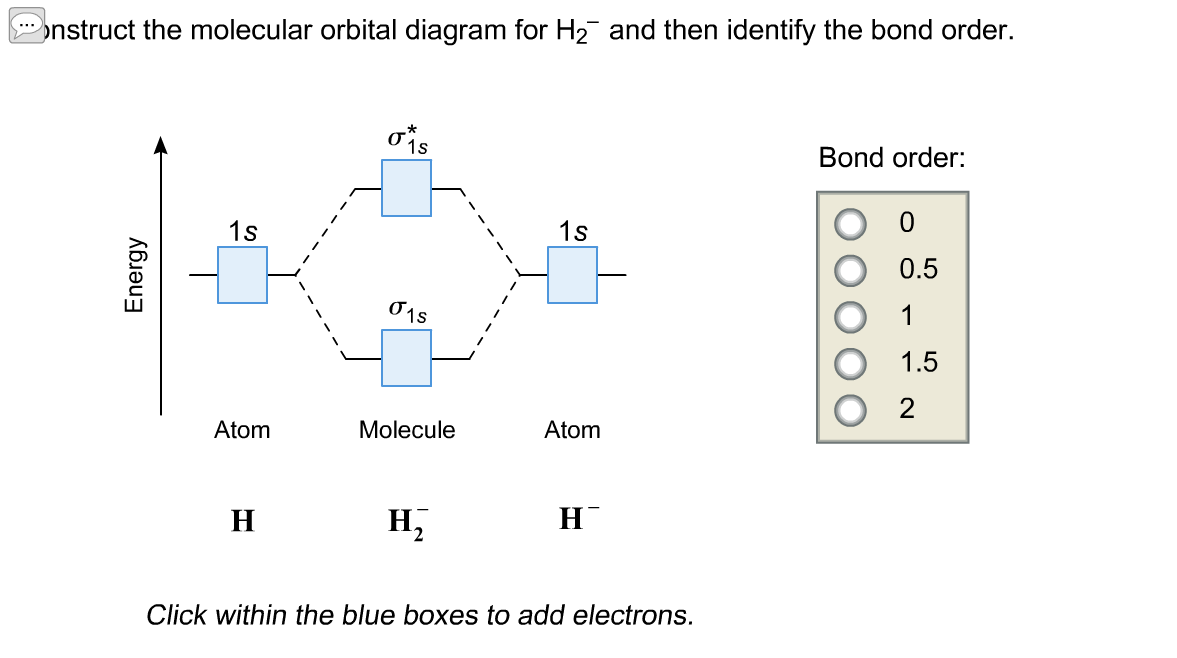
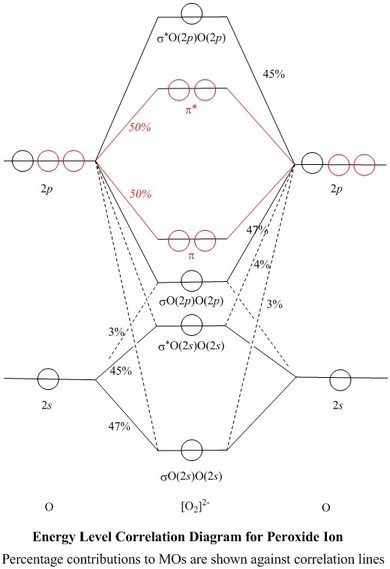
Molecular orbital diagram of h2. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the. Molecular orbitals of h2 and he2. The molecular orbital energy level diagram for the h2 ion. Suppose that the ion is excited by light so that an electron moves from a lower energy to a higher energy molecular orbital. πε and jr k r mr lr defined explicitly in atkins. Sketch the molecular orbitals of the h2 ion and draw its energy level diagram. scheme known as Linear combination of atomic orbitals (LCAO). As per LCAO, molecular orbitals are formed by combination of the atomic orbitals of the combining atoms. The number of molecular orbitals formed must always be equal to the number of atomic orbitals that are combined. The electronic wave functions, namely ψ 1 and ψ 2 for the combining atoms can interact in a constructive or. Question: Each of the following five statements concerns the molecular orbital diagram for H2-. Indicate whether each statement is true or false. This problem has been solved! See the answer See the answer See the answer done loading.
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in 0:15 Molecular Orbital Diagram of Hydrogen Molecule1:39 Molecular Orbital Diagram of Helium Molecule2:54 Molecular Orbital Diagram of Lithium Molecule4:00 Mo... Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi... chemical bonding - chemical bonding - Molecular orbitals of H2 and He2: The procedure can be introduced by considering the H2 molecule. Its molecular orbitals are constructed from the valence-shell orbitals of each hydrogen atom, which are the 1s orbitals of the atoms. Two superpositions of these two orbitals can be formed, one by summing the orbitals and the other by taking their difference.
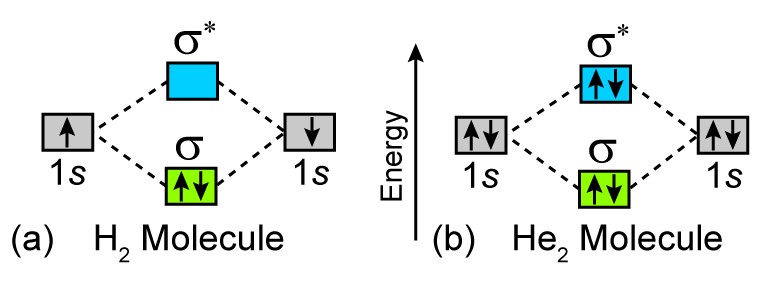
Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbitals of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbitals are excluded from the region between the nuclei and hence the higher the energy the resulting molecular. The basic tenant of Molecular Orbital Theory (MO Theory) is that the number of MOs formed by a linear combination of atomic orbitals (LCAO) is equal to the number of AOs used. The energy splitting caused by electron/electron repulsion generates two MOs due to the one #1s# orbital per hydrogen that is bonding. Draw the molecular orbital energy level diagram of H2. Answer. Prev Question Next Question. Related Questions to study. The degenerate orbitals of. The metal d-orbitals that are directly facing the ligands in K 3... Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+.
Discussed in this video are. Draw mo energy diagrams for the molecular ions h2 and h2. Construct the molecular orbital diagram for h2. When the 1s wave functions of the two ceh atoms are linearly combined we get a sigma s bonding orbital denoted as s 1s in the diagram herethis approach is called linear combination of atomic orbitals lcao.
Molecular orbitals of H. The simplest neutral molecule is molecular hydrogen, H 2, which consists of two electrons and two protons. The molecular orbital Hamiltonian in this case is the same as it is for the molecular hydrogen ion and the molecular orbitals are the same as for the molecular ion. HH2 mo =− ℏ2 2me ∇2− e2 4πϵ0|.
is called Molecular Orbital Theory. • MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule. • Molecular orbitals are constructed by taking linear combinations of the valence orbitals of atoms within the molecule. For example, consider H2:
of the orbitals is specified. b. Molecular Orbital Picture We are now in a position to discuss the basic principles of the molecular orbital (MO) method, which is the foundation of the electronic structure theory of real molecules. The first step in any MO approach requires one to define an effective one electron Hamiltonian, hˆ eff. To this.
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me.
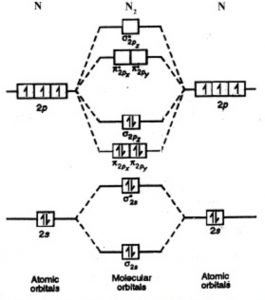
In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons. The bond order of diatomic nitrogen is three and it is a diamagnetic molecule. Construct the molecular orbital diagram for h2 and then identify the bond order. According to mot number of atomic orbitals combined.
Chemical bonding molecular orbitals of h2 and he2. Bonding mos antibonding mos and bond order. One of the molecular orbitals in this molecule is constructed by adding the mathematical functions for the two 1s atomic orbitals that come together to form this molecule. Draw mo energy diagrams for the molecular ions h2 and h2.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
Please note the diagram is for He2 + but the He-H is very similar answered Mar 21 '13 at The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbital s, for the H2 molecule is shown in Figure For the molecule He2: a) Draw the molecular orbital diagram. A molecular orbital explicitly.
38 molecular orbital diagram of h2 Written By Chelsea P. Mariano. Monday, November 8, 2021 Add Comment Edit. 3 Feb 2021 — For H2, bond order = 1/2 (2-0) = 1, which means H2 has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule. Again, in.
1. Problem: Draw MO energy diagram s for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. How to write simple Molecular Orbital Diagram s and.
Molecular orbital diagram of h2. When creating the molecular orbitals from the p orbitals notice the three atomic orbitals split into three molecular orbitals a singly degenerate σ and a doubly degenerate π orbital. Molecular orbitals of h2 and he2. Bonding mos antibonding mos and bond order. Evaluate the ground state electronic energy based.
In order to predict the bond order molecular orbital diagram for h2 is to be drawn. The molecular orbital energy level diagram for the h2 ion. The bond order of diatomic nitrogen is three and it is a diamagnetic molecule. Construct the molecular orbital diagram for h2 and then identify the bond order. Consult a diagram of electron orbital shells.
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical.
Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic. Spin‐orbitals of type 1 and 3 have the same symmetry, and therefore can "mix" (to give improved wavefunctions and energy eigenvalues): 1 ψψ αβ...
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can.













0 Response to "37 Molecular Orbital Diagram Of H2"
Post a Comment