37 Match The Appropriate Octahedral Crystal-field Splitting Diagram Fe4+
Reaction of U(SPri)4 with [NEt3H][BPh4] in the presence of hexamethylphosphoric triamide (hmpa) afforded the cationic compound [U(SPri)2(hmpa)4][BPh4]2 which presents a trans-octahedral crystal. Match The Appropriate Octahedral Crystal Field Splitting Diagram Fe4 Cobalt 3 has 6 d electrons cobalt normally has 9 valence electrons but youve lost 3. Match the appropriate octahedral crystal fi…
W9.1 Jahn-Teller Effect 75 W9.2 Examples of Weak and Strong Crystal Field Effects 75 W9.3 Crystal Fields and Cr3C in Al2 O3 75 W9.4 Experimental Results for in the Free-Spin Limit 78 W9.5 Spin Glasses and the RKKY Interaction 79 W9.6 Kondo Effect and s-d Interaction 79 W9.7 T for Ni 80 xii WEB CONTENTS
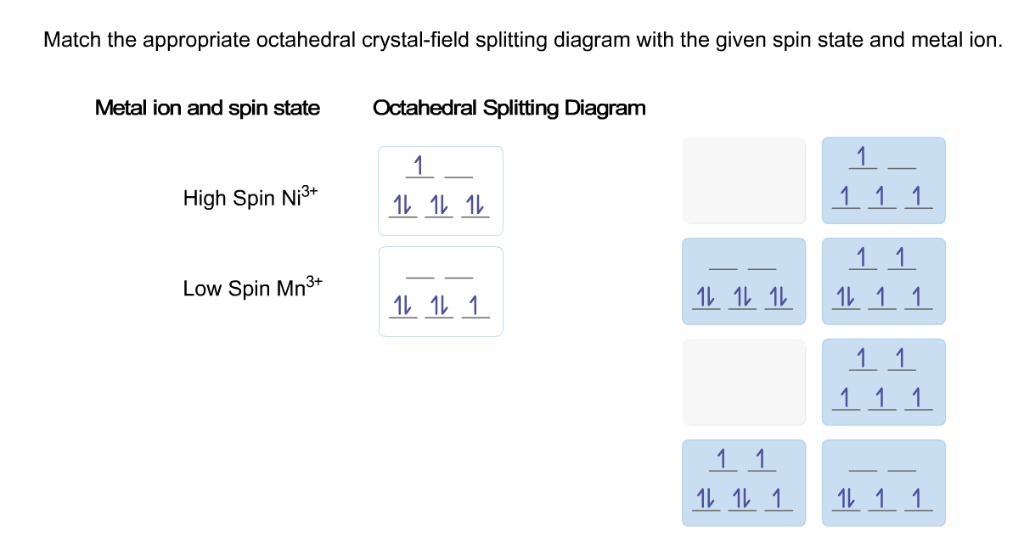
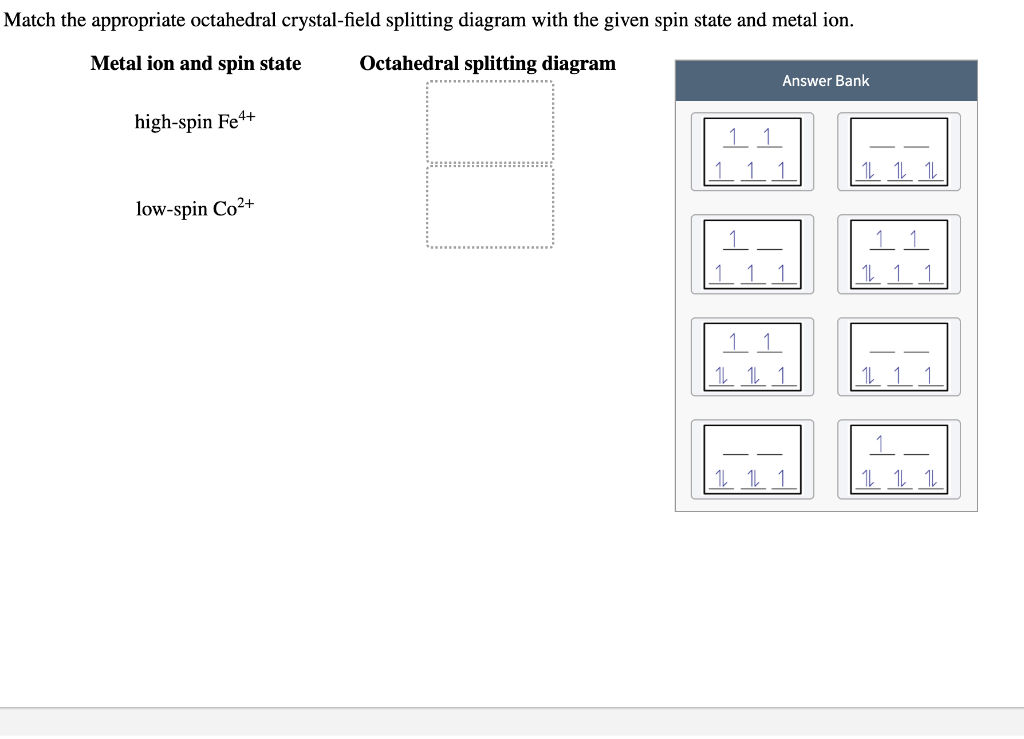
Match the appropriate octahedral crystal-field splitting diagram fe4+
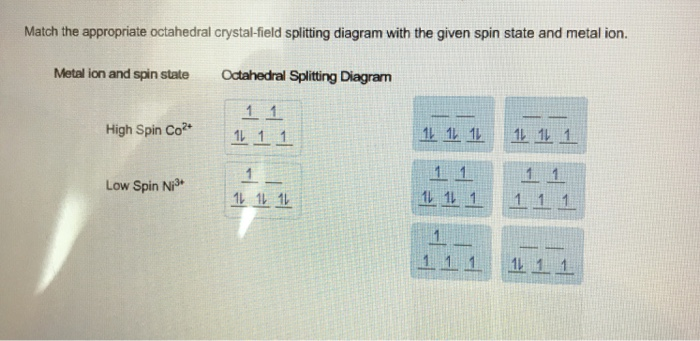
Crystal-electric field splitting and the symmetry of 3d orbitals (a) CEF splitting at tetrahedral and octahedral lattice sites. Due to the lattice symmetry and shape of the orbitals, the splitting at the octahedral sites is significantly larger. In tetrahedral symmetry the ground state is labelled e. Chemistry questions and answers. Question 17 of 25 > Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4+ low-spin Mn2+ 11. Co2+ ions in an octahedral crystal field stabilize a jeff = 1/2 ground state with an orbital degree of freedom and have been more recently put forward for realizing Kitaev interactions [4], a prediction we have tested by investigating spin dynamics in two cobalt honeycomb lattice compounds, Na2Co2TeO6 and Na3Co2SbO6, using inelastic neutron.
Match the appropriate octahedral crystal-field splitting diagram fe4+. Co2+ ions in an octahedral crystal field stabilize a jeff = 1/2 ground state with an orbital degree of freedom and have been more recently put forward for realizing Kitaev interactions [4], a prediction we have tested by investigating spin dynamics in two cobalt honeycomb lattice compounds, Na2Co2TeO6 and Na3Co2SbO6, using inelastic neutron. Spin crossover in (Mg,Fe3+)(Si,Fe3+)O3 bridgmanite: effects of disorder, iron concentration, and temperature. NASA Astrophysics Data System (ADS) Shukla, Gaurav; Wentzcovitch, Renata. The spin crossover of iron in Fe3+-bearing bridgmanite, the most abundant mineral of the Earth's lower mantle, is by now a well-established phenomenon, though several aspects of this crossover remain unclear. Match The Appropriate Octahedral Crystal Field Splitting Diagram Which of the following are true for the crystal field model of an octahedral complex ion. As a result the splitting observed in. Given below are some important topics of Physical, Organic and Inorganic that require special attention: Physical: ∑ Bohr's theory of atomic structure, quantum numbers and orbitals. ∑ MO approach to diatomic molecules, concepts of hybridization/VSEPR theory. ∑ Van der Waals equation of state and its application to the behaviour of real.
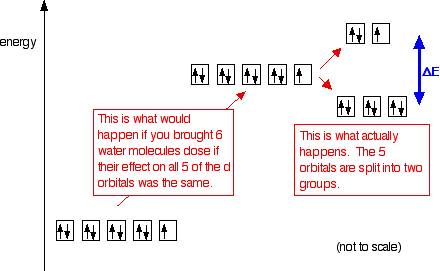
Solid State ChemiStry and itS appliCation 2014 Anthony R. West Crystal-electric field splitting and the symmetry of 3d orbitals (a) CEF splitting at tetrahedral and octahedral lattice sites. Due to the lattice symmetry and shape of the orbitals, the splitting at the octahedral sites is significantly larger. In tetrahedral symmetry the ground state is labelled e. Fig.9.8: d orbital splitting in an octahedral crystal field The crystal field splitting, Δ↓o, depends upon the field produced by the ligand and charge on the metal ion. Some ligands are able to produce strong fields in which case, the splitting will be large whereas others produce weak fields and consequently result in small splitting of d. Match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. The splitting diagram for square planar complexes is more complex than for octahedral and tetrahedral complexes and is shown below with the relative energies of each orbital. As a result the splitting observed in a tetrahedral crystal field.
24 THE CRYSTAL FIELD 13 Other Geometries In 4 coordination, two geometries are common, tetrahedral and square planar, for which the crystal eld splitting patterns are shown in Fig. 1.4. For the same ligand set, the tetrahedral splitting parameter is smaller than that for the octahedral geometry by a factor of 23 because we now have only four. A huge enhancement in the saturation (Ps), the remnant polarization (Pr), and the coercive electric field (Ec) of the sample La0.98 0.02FeO3 by 7.5, 24, and 12 times, respectively, when compared. Thus the size of the octahedral gaps differs in the different structures and therefore the crystal fields induced by a the oxygen dianions too. 2-By means of the shorter distance O - metal in ruby, the octahedral crystal field of ruby is stronger than that of chromium oxide. This leads to a larger splitting of the t2g eglevels in ruby. Orgel Diagrams 26.16 Racah Parameters 26.24 Terms Correlation Diagrams under the Effect of Weak and Strong Field Effects 26.26 Tanabe-sugano Diagrams (T-S Diagram) 26.29 Charge-Transfer Transitions 26.34 Types of Magnetism 26.39 Summary 26.55 Solved Examples 26.56 Exercises 26.57 27.
Match the appropriate octahedral crystal field splitting diagram fe4. This theory has been used to describe various spectroscopies of transition metal coordination complexes in particular optical spectra colors. Cobalt 3 has 6 d electrons cobalt normally has 9 valence electrons but youve lost 3. Crystal field stabilization energies for.
S denotes the ground-state spin and J is the (weak-crystal-field) effective total angular momentum into which the substitutional ground-state term is split by spin-orbit interaction in first order. The J > 3/2 of ' E is further split in second order.
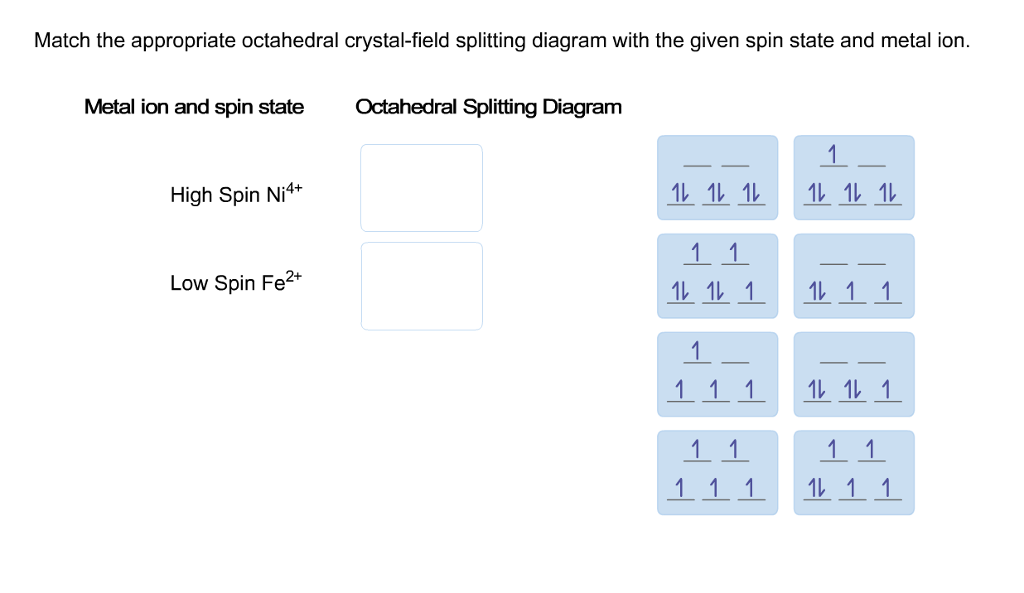
Chemistry. Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Fe4+. Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state.
Match The Appropriate Octahedral Crystal Field Splitting Diagram. There is a large energy separation between the d z² orbital and the d xz and d yz orbitals meaning that the crystal field splitting energy is large. Match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion.
Match the appropriate octahedral crystal field splitting diagram match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. Because none of the d orbitals points directly at the ligands in a tetrahedral complex these complexes have smaller values of the crystal field splitting energy δ t.
Match the appropriate octahedral crystal field splitting diagram. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level.
Match the appropriate octahedral crystal field splitting diagram fe4. A left handed propeller will pull the stern to starboard right when in reverse. Match the appropriate octahedral crystal field splitting diagram. A calculate the crystal field splitting energy δ in kjmol. It depends on the identity of the metal ion the charge on this ion and.
Match The Appropriate Octahedral Crystal Field Splitting Diagram Fe4 Crystal field stabilization energies for octahedral complexes four coordinate geometries crystal field theory ffqppor tetrahedra…
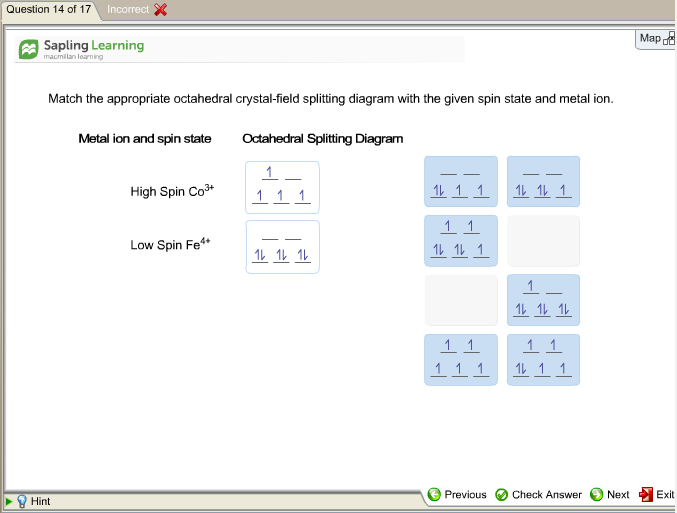
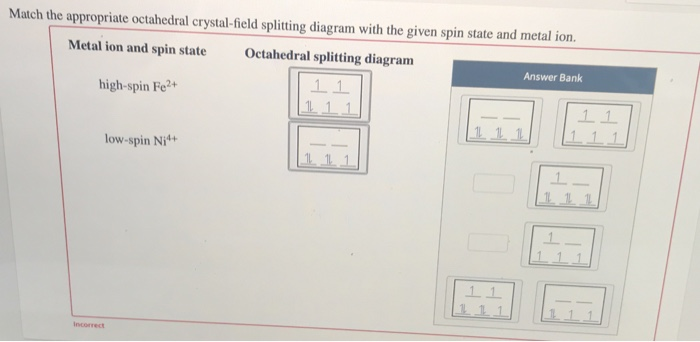
Ans. If the element present in high spin.. View the full answer. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4+ 1 1 1 1 1 1 1 1 low-spin Co2+ 1 1 1 1 1 1 11 1 1 1 1 1 1 1 1 1 1.
Figure B-1 shows a band-model energy-level diagram of corundum and some of its trace elements. In this model, the energy of an electron (in electron volts) is plotted on the vertical axis. The extent of the horizontal axis is meant to imply only that the corundum energy levels are the same everywhere in the crystal.
This problem has been solved! See the answer. See the answer See the answer done loading. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting DiagramHigh Spin Mn2Low Spin Fe4. Show transcribed image text.
Academia.edu is a platform for academics to share research papers.
Question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. Match the appropriate octahedral crystal field splitting diagram. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level.
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the.
Chemistry questions and answers. Question 17 of 25 > Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4+ low-spin Mn2+ 11.
This problem has been solved! See the answer. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Show transcribed image text.
Match the appropriate octahedral crystal field splitting diagram. A none of the 3d orbitals point directly at ligands b t2 orbitals are more stable than e orbitals c a small crystal field splitting energy results in a paramagnetic complex d the low spin case gives maximum unpaired electrons e for a given ligand.














0 Response to "37 Match The Appropriate Octahedral Crystal-field Splitting Diagram Fe4+"
Post a Comment