37 Bohr Diagram For Sodium
Bohr Diagrams • A Bohr diagram is a diagram that shows how many _____ are in each shell surrounding the nucleus. • Named in honour of _____, a Danish physicist who developed several models for showing the arrangement of electrons in atoms. • There are three main background questions to explore before we start drawing Bohr diagrams. Bohr Diagram Of Sodium. Photo by. Bert Hardy. on . Getty Images · Bohr Diagram Of Sodium. Christy Sego. 115 followers. Bohr model of hydrogen atom, postulates, energy levels, calculation of radius, velocity, emission or absorption energy of electron by Bohr's theory.
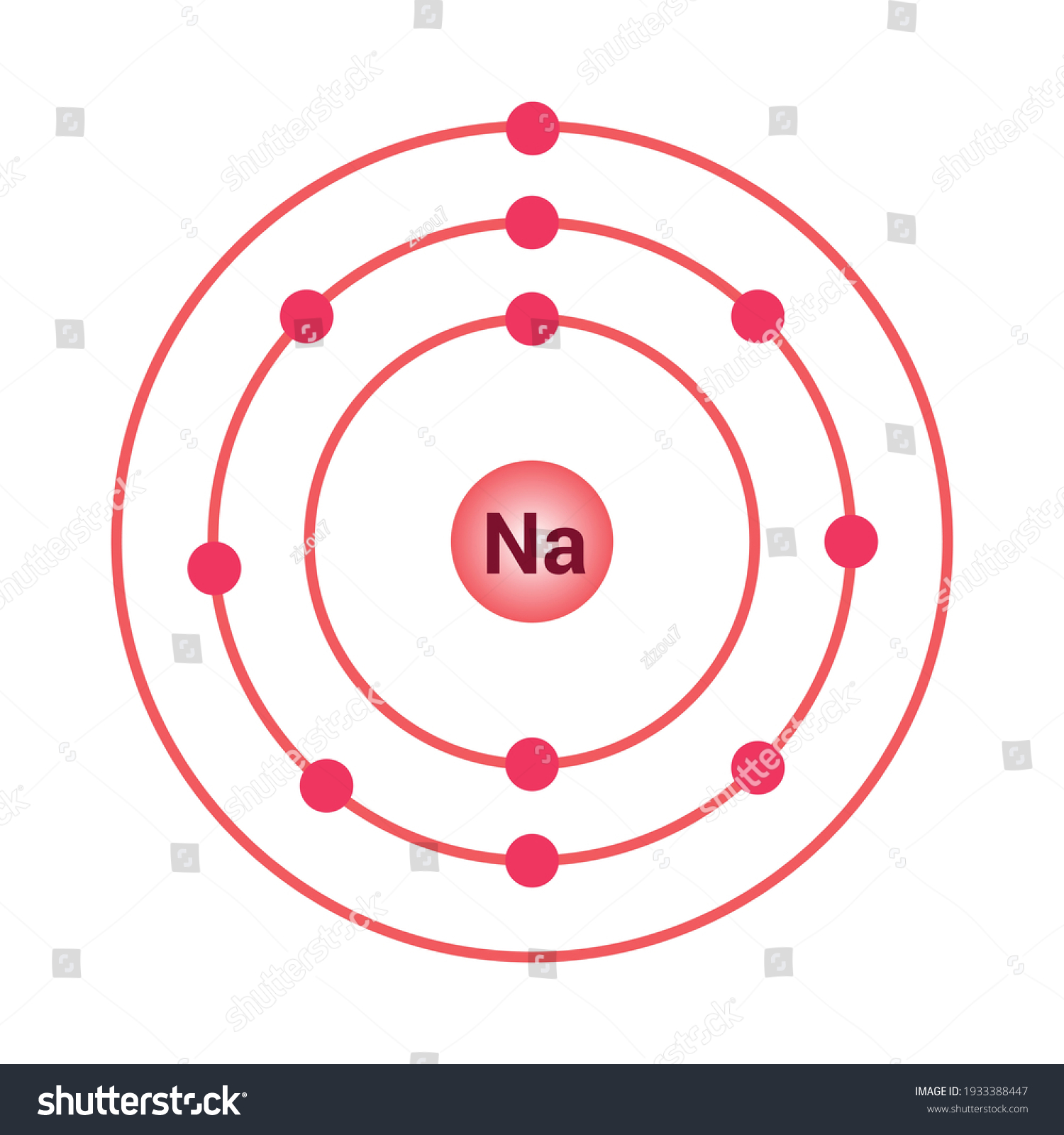
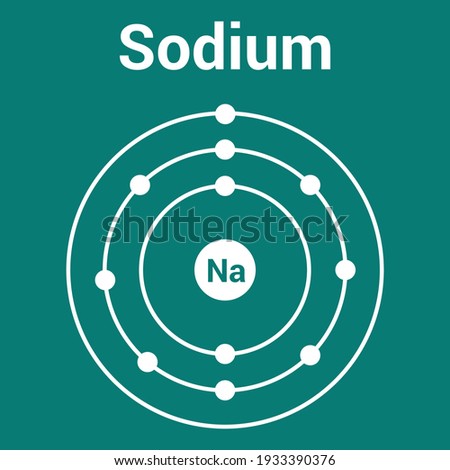
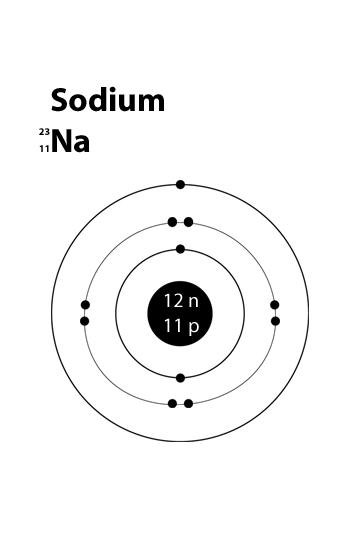
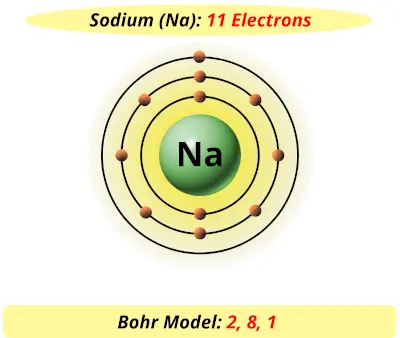
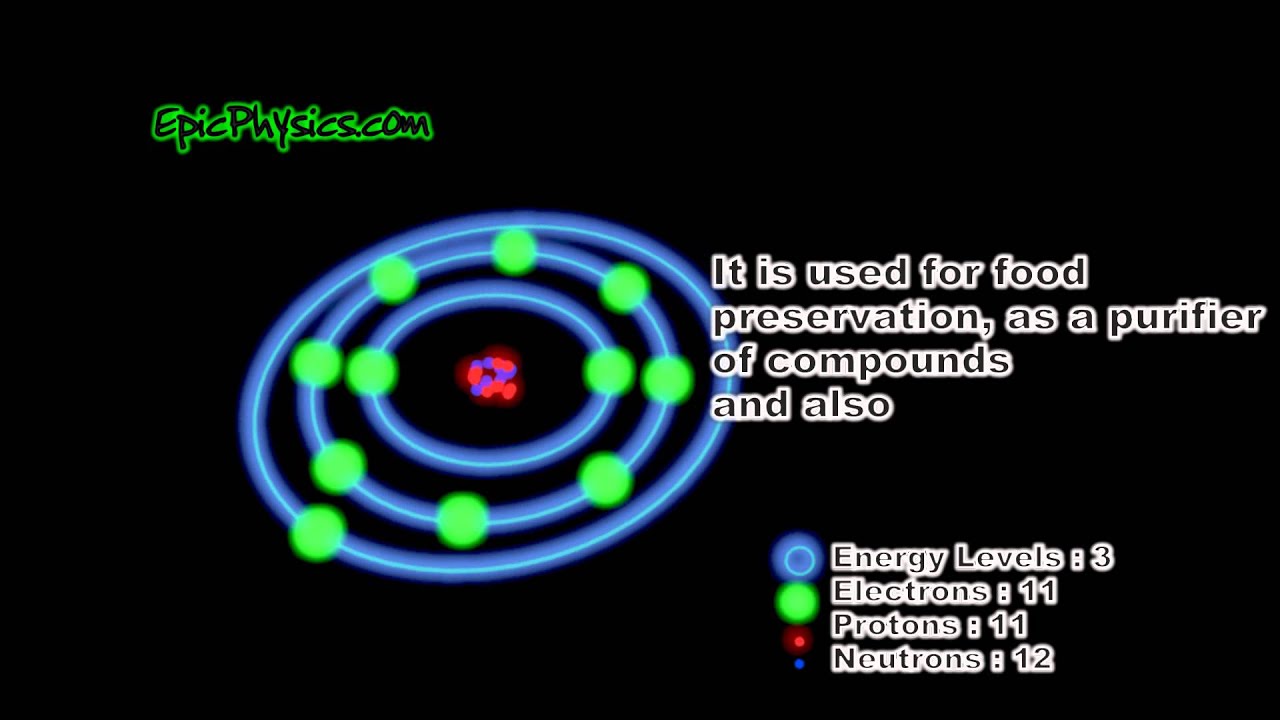
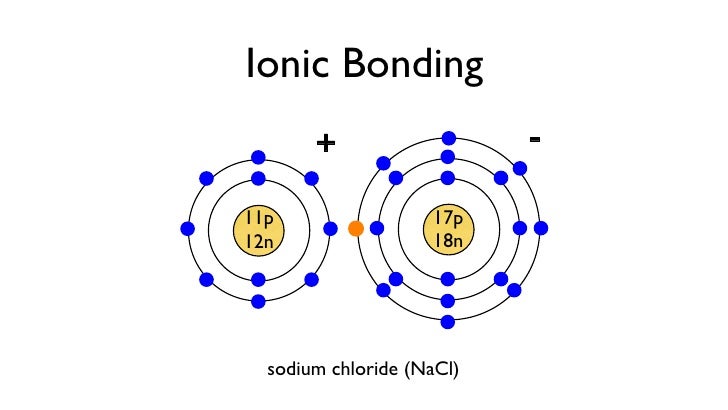
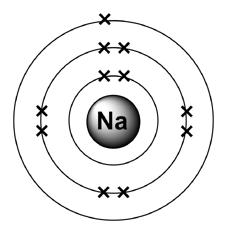
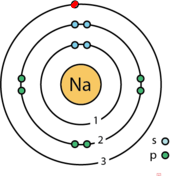
The Bohr Model of Sodium (Na) has a nucleus that contains 12 neutrons and 11 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sodium contains only 1 electron that also called valence electron. Name. Sodium Bohr Model.

Bohr diagram for sodium
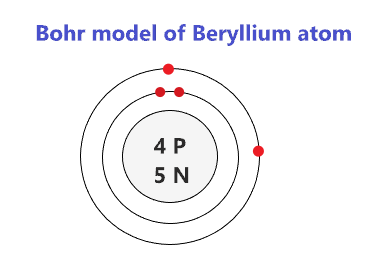
Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8. What is the bohr Rutherford diagram of sodium? a nucleus with 11 p, 12n with 3 shells. the 1st shell has 2e's, the 2nd has 8e's, and the 3rd has only 1e Can you draw a Bohr model of magnesium?
Bohr diagram for sodium. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing. To draw the Bohr diagram for "NaCl", we should first draw the individual diagrams for both "Na" and "Cl". The atomic number of "Na" is 11, so it has 11 electrons. Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The Bohr model of Helium is drawn with only one electron shell and it contains 2 electrons. Helium is neutral and its atomic number is 2, hence, the number of protons and electrons available for its Bohr diagram is also 2. The number of neutrons for the Bohr diagram of Helium can be founded by subtracting the number of protons from the atomic.
Bohr Diagram Of Sodium. Bohr Model Of Sodium Pdfshare. Bohr Model Sodium Atom Chemistry Rutherford Model Png. Ppt Atomic Structure Powerpoint Presentation Id5055482. Using The Aph Periodic Table To Determine Valence. Bohr Rutherford Diagram For Sodium. Sodium hydroxide is also known as lye or soda, or caustic soda. At room temperature, sodium hydroxide is a white crystalline odorless solid that absorbs moisture from the air. It is a synthetically manufactured substance. Diagrams - Public Service Announcement: Sodium Fluoride. Lewis Dot Diagram- The sodium transfers one electron to the fluorine so they can both become stable. Bohr-Rutherford Diagram: Sodium is the cation as it has a positive charge after losing one electron, while fluoride is the anion as it has a negative charge after gaining one electron. In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the solar system Joseph Larmor model (1897), the cubical model (1902.
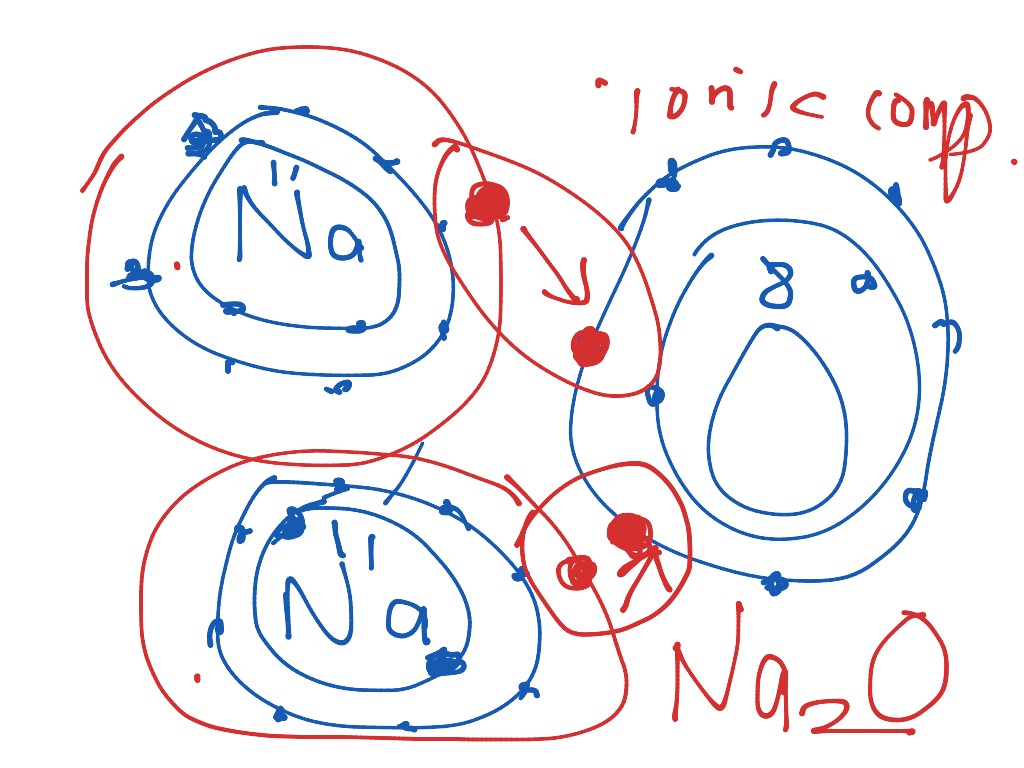
NaCl, sodium chloride, is an IONIC compound, which means electrons are TRANSFERRED from one atom to another.You'll have to draw the Bohr-Rutherford Diagram o... The bohr Rutherford diagram for oxygen h as 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. Since Sodium's Atomic Number is 11, that is also the number of electrons. The first energy level can hold 2 electrons, the next 8, and the third So the diagram has two ele ctrons on the first level, eight on. Example: Determine the formula of a compound formed by the reaction of sodium and fluoride. Solution: First examine the electron arrangement of the sodium and fluorine atoms. Symbol: Atomic No. Bohr diagram: Group No. Lewis Dots: Na: 11: 2 - 8 - 1: 1: 1: F: 9: 2 - 7: 7: 7: Write the Lewis symbol for each atom. See Graphic on the left. Below is an illustration of the Bohr model of a sodium atom. If you look at the diagrams of the sodium and chlorine atoms you can see that sodium normally has. Bohr-Rutherford diagram of sodium chloride? Bohr-Rutherford diagram of The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2.
Bohr Diagrams in terms of A Bohr diagram is a diagram that shows how many each shell surrounding the nucleus. Named in honour of , a Danish physicist who developed several models for showing the arrangement of electrons in atoms. There are three main background questions to explore before we start drawing Bohr diagrams.
30 seconds. Q. What best describes the difference between the bohr model and the lewis dot? answer choices. The bohr model diagram represents all the subatomic particles, while the lewis dot diagram only shows the symbol and the valence electrons. The lewis dot shows all the electrons and the bohr model only shows the electrons in the last shell.
Check me out: http://www.chemistnate
Sodium has 2 electrons in its first shell, 8 in its second and 1 in its third.Check me out: http://www.chemistnate
Lewis Symbols. Sodium chloride is the chemical name for salt. How do you draw a Bohr diagram for a sodium atom? Bohr diagram for calcium chloride swim directv wiring diagram 30 model a wire diagram 1984 chevy p 32 wiring schematic compaq power supply wiring diagram 2008.
sodium atom sodium ion argon atom chlorine atom chlorine ion potassium atom potassium ion Atomic number Number of 10 protons 10 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3.
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, : Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.
The shell closest to the nucleus is called the K.Bohr rutherford diagram of aluminum further atomo furthermore file 20 calcium ca enhanced bohr model along with bohr model drawing of oxygen in addition bohr rutherford diagram for aluminum in addition sodium lab eng moreover bohr rutherford diagram as well as electron shell diagram.
What is the bohr Rutherford diagram of sodium? a nucleus with 11 p, 12n with 3 shells. the 1st shell has 2e's, the 2nd has 8e's, and the 3rd has only 1e Can you draw a Bohr model of magnesium?
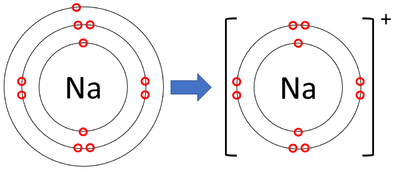
The sodium atom wants to lose an electron and the chlorine atom wants to gain an electron. When the two atoms come together the electron from the sodium atom jumps into the gap in the outer shell of the chlorine atom. If you look at the diagram the sodium. ion. now contains only ten electrons and the new chloride ion (an anion) has eighteen.
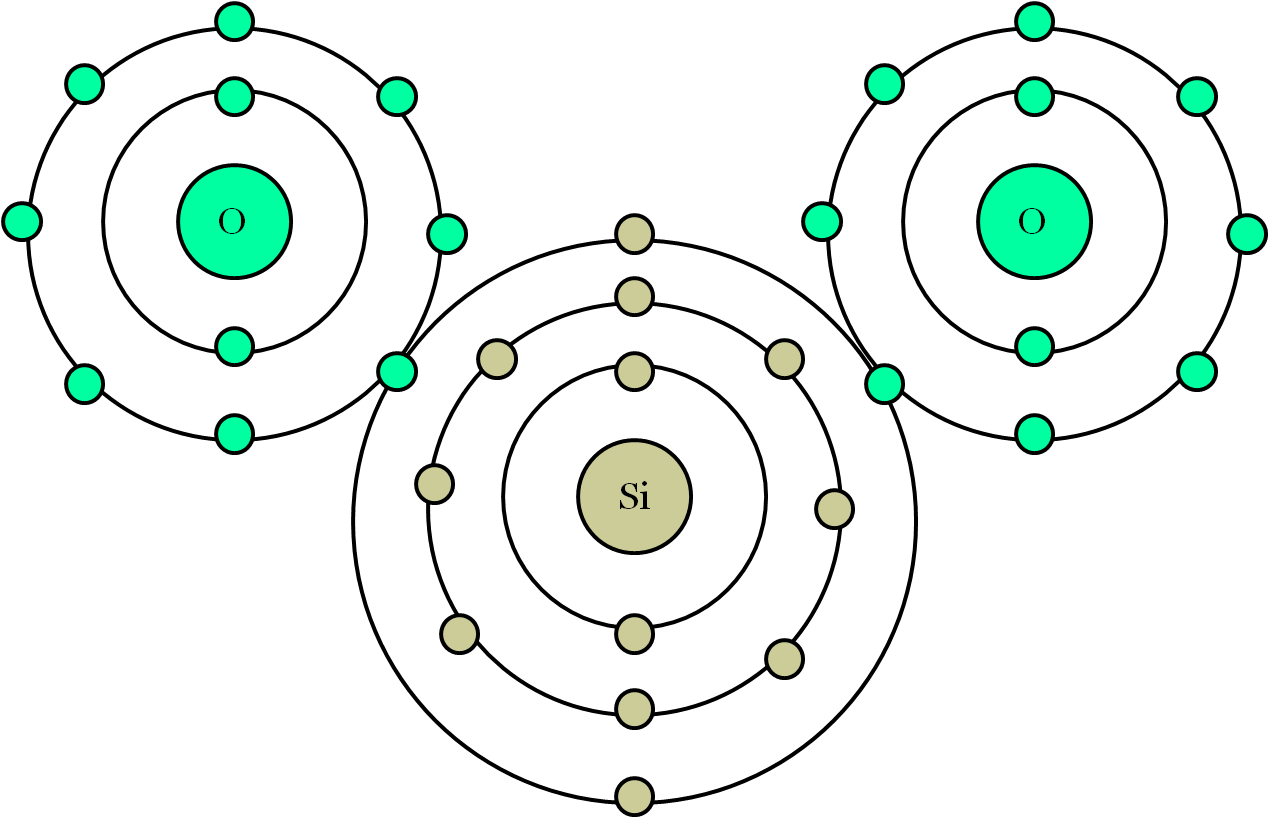
B. Compare a Bohr diagram and a Lewis diagram. Explain how they are: similar. different. Draw the Bohr Model diagram for each of the following atoms. Neon Atom Fluorine atom Fluorine Ion Sodium Atom Sodium Ion Draw the Bohr model diagram for each of the following compounds. Carbon Dioxide (CO2) Ammonia (NH3) Calcium Chloride (CaCl2)
Snc2d bohr diagram worksheet name: • there are three main background questions to explore before we start drawing bohr diagrams. Bohr diagrams • a bohr diagram is a diagram that shows how many _____ are in each shell surrounding the nucleus. Snc1p bohr diagram worksheet name: Some answers are provided for you. Drawing dohr model diagrams 1.
Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8.
Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, well show a sample Bohr diagram for hydrogen. H Hydrogen. 0 neutrons. You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom.
Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale.
The Element Sodium-- Sodium Atom. Sodium is a chemical element in the periodic table that has the symbol Na (Natrium in Latin) and atom number 11. Sodium is a soft, waxy, silvery reactive metal belonging to the alkali metals that is abundant in natural compounds (especially halite). Keeping this in view, what is a Bohr diagram?














0 Response to "37 Bohr Diagram For Sodium"
Post a Comment