40 nf molecular orbital diagram
collegedunia.com › exams › hydrogen-spectrum-seriesHydrogen Spectrum: Series, Line Spectrum Hydrogen, Wavelength Apr 27, 2022 · Since both nf and ni are integers, this immediately shows that in transitions between different atomic levels, light is radiated in various discrete frequencies. For the hydrogen spectrum, the results of the Bohr’s model suggested the presence of other series spectra for hydrogen atoms corresponding to transitions resulting from nf=1 and ni=2 ... Coumarinolignans with Reactive Oxygen Species (ROS) and NF-κB ... A molecular formula of C 20 H 18 O 8 with twelve degrees of unsaturation was established on the basis ... the activation of NF-κB leads to the up-regulation of pro-inflammatory genes such as ... software was used for visualization of the molecular orbitals (isovalue = 0.03) involved in UV and ECD transitions. 3.5. Assessment of ROS Inhibition ...
Trigonal Pyramidal & Bipyramidal in Molecular Geometry - Study.com Learn about trigonal pyramidal and bipyramidal molecular geometry. ... while Nitrogen trifluoride, {eq}NF_3 {/eq}, has a bond angle of 102 degrees. ... of an s orbital, three p orbitals, and a d ...
Nf molecular orbital diagram
Conducting Polymer-Based Nanofibers for Advanced ... - SpringerLink According to the theory of quantum mechanics, delocalization occurs when molecular orbitals in conjugated polymer overlap with each other. In the presence of an external electric field, electrons will break away from the valence band or main chain structure, and an electric current will be formed inside the polymer, thus possessing excellent ... Atomic orbital - Wikipedia The shapes of the first five atomic orbitals are: 1s, 2s, 2p x, 2p y, and 2p z. The two colors show the phase or sign of the wave function in each region. Each picture is domain coloring of a ψ (x, y, z) function which depend on the coordinates of one electron. Molecular Geometry: A Complete Overview - PSIBERG The lewis diagram of a molecule (NH3) can be drawn by following the step below; Count the total number of valence electrons in a molecule. The valence electrons of nitrogen and the fluorine atoms are added and the number comes out is 26. N + H + H + H Valence electrons = 5 + 7 + 7 + 7 = 26 Choose the central atom
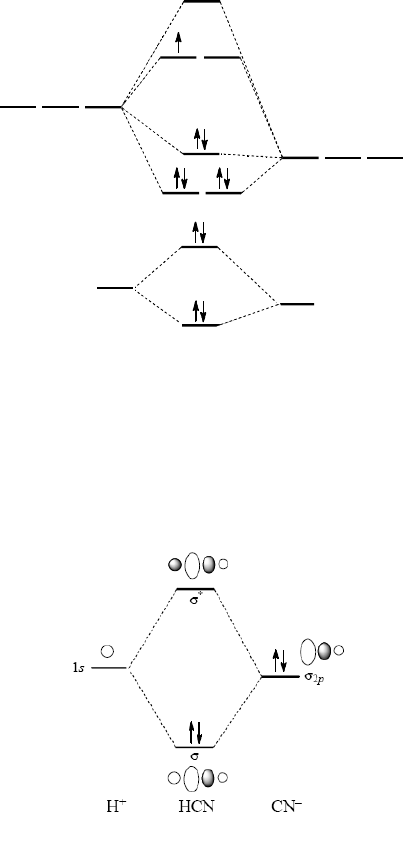
Nf molecular orbital diagram. en.wikipedia.org › wiki › Lone_pairLone pair - Wikipedia The s character rich O σ(out) lone pair orbital (also notated n O (σ)) is an ~sp 0.7 hybrid (~40% p character, 60% s character), while the p lone pair orbital (also notated n O (π)) consists of 100% p character. Both models are of value and represent the same total electron density, with the orbitals related by a unitary transformation. An introduction to molecular orbital theory - Stemco The bond order of a molecule can be calculated by the following equation: Bond order =0.5* (number of electrons in bonding MOs - number of electrons in antibonding MOs) In diatomic hydrogen, the two hydrogen 1s AOs combine to form a bonding and antibonding MO. According to LCAO, n number of AOs combine to form n number of MOs. HCN Lewis Structure, Molecular Geometry, Shape, and Polarity Carbon forms one single bond with the Hydrogen atom and forms a triple bond with the Nitrogen atom. HCN has a total of 10 valence electrons. It is covered under AX2 molecular geometry and has a linear shape. The bond angles of HCN is 180 degrees. Hydrogen Cyanide is a polar molecule. M-polynomial and neighborhood M-polynomial methods for topological ... A molecular structure can be described by a parameter, polynomial or matrix. The physico-chemical features, bio-activities of a chemical compound are predicted using the numerical descriptor called topological index. Topological indices remodel the data of molecular graphs into numerical characteristics which in turn improvises the discussion of structure property and activity relationship ...
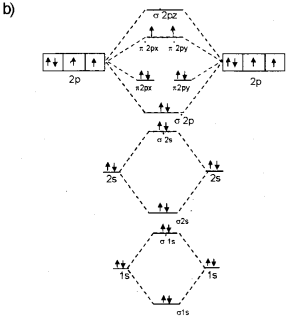
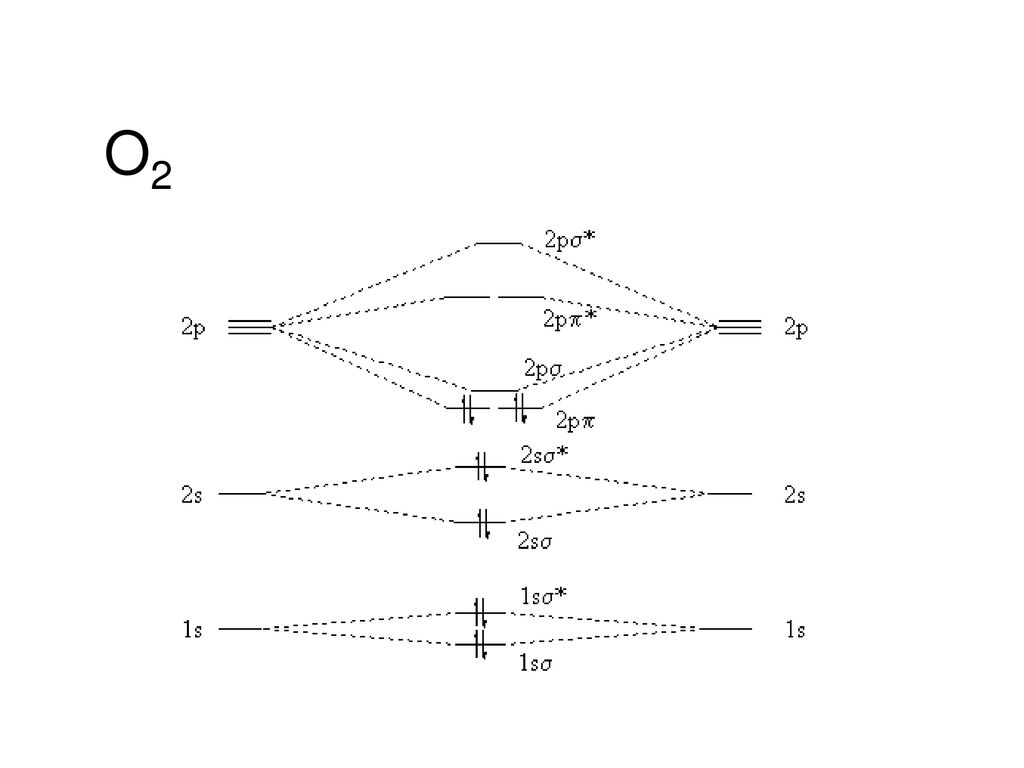
The increasing order of energies of various molecular orbitals of N2is ... Here you can find the meaning of The increasing order of energies of various molecular orbitals of N2is given below:σls <σ*ls <σ2s <σ*2s <π2px = π2py <σ2pz < π*2px= π*2py<σ*2pzThe above sequence is not true for the moleculea)C2b)B2c)O2d)Be2Correct answer is option 'C'. Boosting external quantum efficiency to 38.6% of sky-blue delayed ... The encircled and enlarged parts indicate the n orbital character. On the other hand, the calculated molecular polarity index (MPI) values of these molecules in S 1 and T 1 states are 14.68 to 19.95 and 8.65 to 10.01 kcal mol −1, respectively (table S4). › ncert-solutions-for-class-11NCERT Solutions for Class 11 Chemistry Chapter 4 - LearnCBSE.in Answer: Bonding molecular orbital has lower energy and higher stability. Question 19. Define antibonding molecular orbital. Answer: The molecular orbital formed by the subtractive effect of the electron waves of the combining atomic orbitals, is called antibonding molecular orbital. Question 20. Final Exam Flashcards | Quizlet the bottom orbital of a molecular orbital is the _____ orbital. it has _____ energy and _____ stability bond order = 1/2(number of electrons in bonding molecular orbitals - number of electrons in antibonding orbitals)
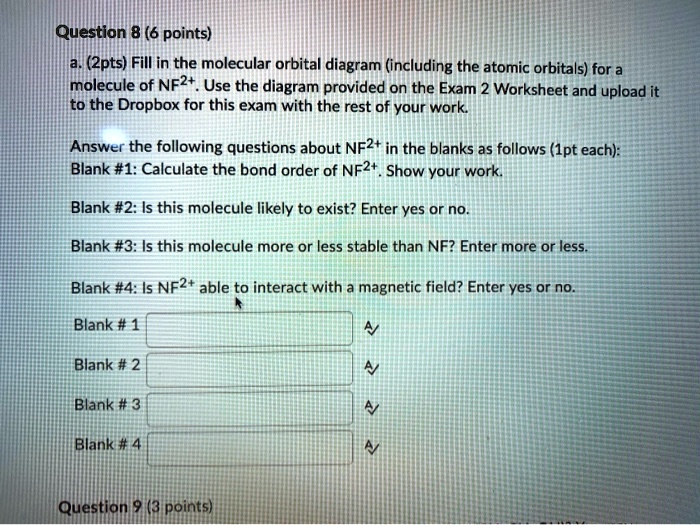
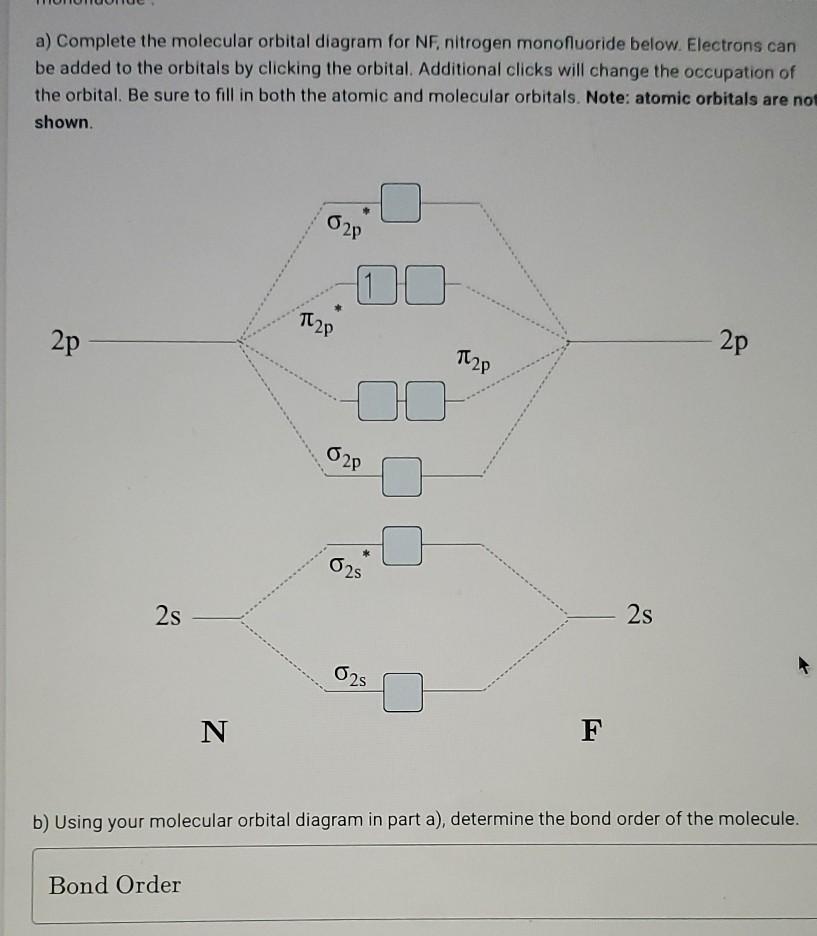
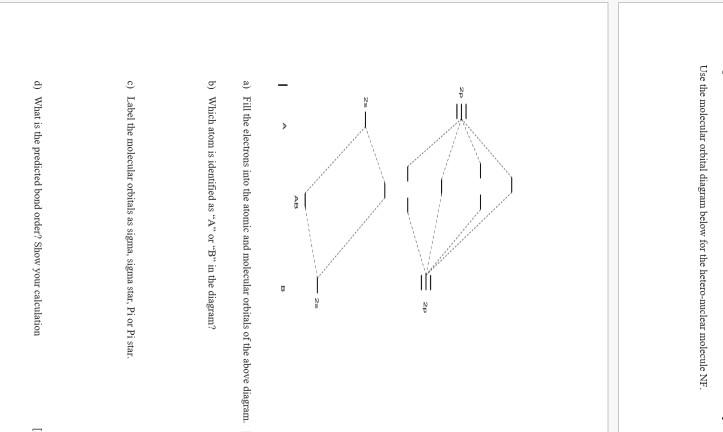
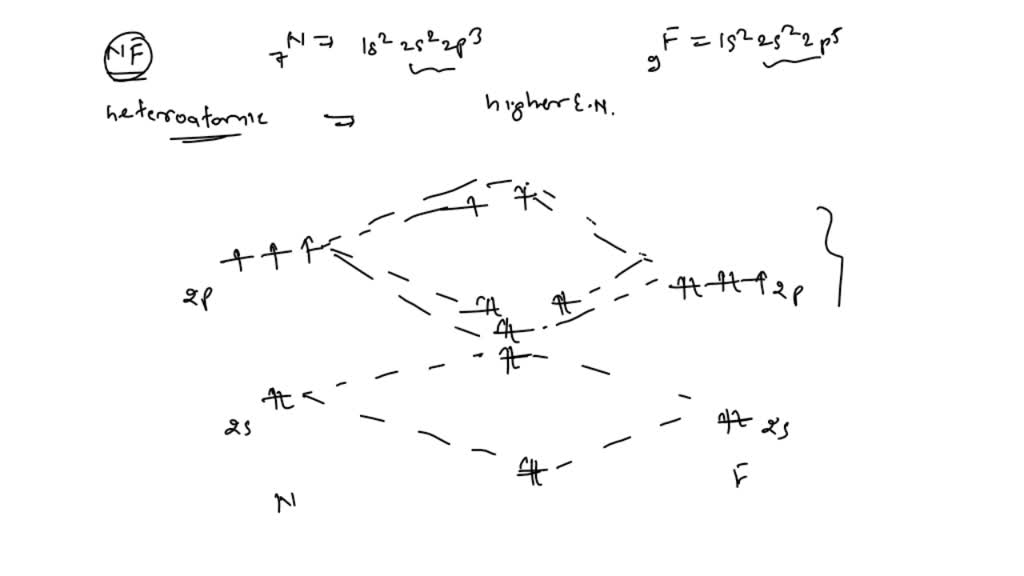
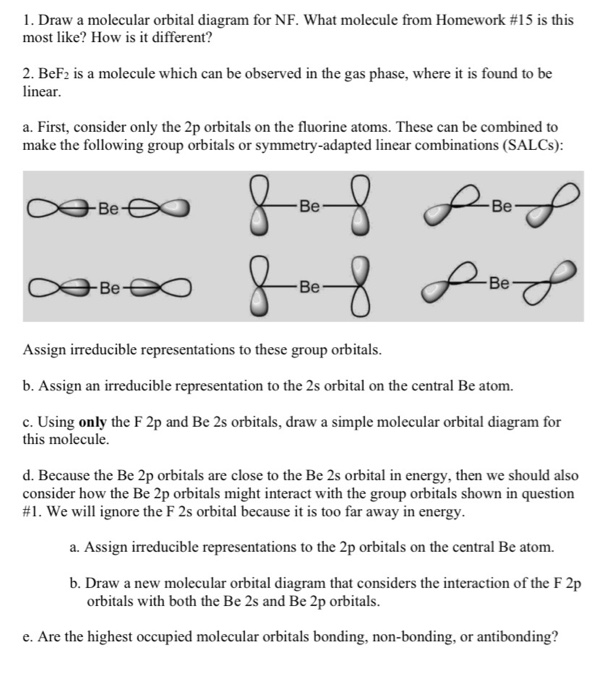
en.wikipedia.org › wiki › Nuclear_thermal_rocketNuclear thermal rocket - Wikipedia Most liquid-fueled chemical rockets use either hydrogen or hydrocarbon combustion, and the propellant is therefore mainly water (molecular mass 18) and/or carbon dioxide (molecular mass 44). Nuclear thermal rockets using gaseous hydrogen propellant (molecular mass 2) therefore have a theoretical maximum Isp that is 3x-4.5x greater than those of ... Draw and label a molecular orbital diagram for nitrogen monofluoride,NF ... Draw and label a molecular orbital diagram for nitrogen monofluoride,NF. Use atleast half a page Give the bond order Give the magnetism, and explain your choice briefly (a few words are enough) If you ionized NF to give NF+, would the bond be shorter or longer? Explain your reasoning briefly (again a few words are plenty) Apr 01 2022 05:38 PM 9.7: Molecular Orbitals - Chemistry LibreTexts Figure is the molecular orbital energy-level diagram for He 2. With a total of four valence electrons, both the σ 1s bonding and antibonding orbitals must contain two electrons. This gives a electron configuration, with a predicted bond order of (2 − 2) ÷ 2 = 0, which indicates that the He 2 molecule has no net bond and is not a stable species. Bond Order Formula: Definition, Calculation, Solved Examples - Embibe Exams The molecular orbital makes the concept of a chemical bond's bond order simple to comprehend. It measures the strength of atom-to-atom covalent connections. Bond order is significant in molecular orbital theory for determining bond strength and is also used in valence bond theory.
SF4 Molecular Geometry, Lewis Structure, Bond Angles and Polarity ( as there are four fluorine atoms, we have to consider valence electrons of all atoms) Total number of valence electrons in SF4 = number of valence electrons in sulfur + number of valence electrons in fluorine = 6 + 28 = 34 valence electrons
9.8: Molecular Orbital Theory - Chemistry LibreTexts Molecular Orbital Diagrams This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
NCERT Exemplar Class 11 Chemistry Unit 4 Chemical Bonding and Molecular ... The types of hybrid orbitals of nitrogen in NO 2+, NO 3- and NH 4+ respectively are expected to be (i) sp, sp 3 and sp 2 (ii) sp, sp 2 and sp 3 (iii) sp 2, sp and sp 3 (iv) sp 2, sp 3 and sp Hydrogen bonds are formed in many compounds e.g., H 2 O, HF, NH 3.
Problem 16: Please answer the following questions about...ask 8 Draw a molecular orbital diagram for NF and calculate the bond order. Also, comment on its magnetic property. May 26 2022 Draw the Lewis structure for NO + . Is the nitrogen- oxygen bond in NO + longer, shorter, or the...
Nitrogen - Wikipedia Molecular orbital diagram of dinitrogen molecule, N 2. There are five bonding orbitals and two antibonding orbitals (marked with an asterisk; orbitals involving the inner 1s electrons not shown), giving a total bond order of three. Atomic nitrogen, also known as active nitrogen, is highly reactive, being a triradical with three unpaired electrons.
What Is The Molecular Geometry Of pf3, if2, of2, ch2o ... - Webnews21 The molecular formula is C2H5OH, with the hydrogen and oxygen in a 2:1 ratio. The molecules have tetrahedral geometry. In other words, each atom has four neighbors to which it is bonded. The four bonds meet at a single point at the center of the tetrahedron. Each bond angle equals 109.5 degrees (in full trigonal planar).
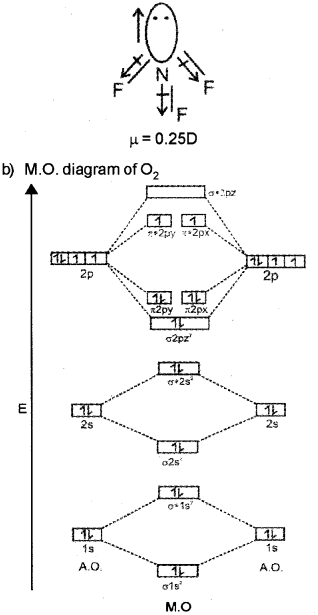
NCERT Exemplar Class 11 Chemistry Chapter 4 Chemical Bonding and ... Q39. The energy of σ2p z: molecular orbital is greater than 2p x and 2p v molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behaviour of the following species: ... NF 3 and CHCl 3. Sol: (i) Dipole moment plays ...
Draw a molecular orbital diagram for NF and calculate the ... - Quesba 2 Answers THE PRIMARY STEPS IN DEVELOPING MO DIAGRAMS Find each atom's valence electron configuration in the molecule. Determine whether the molecule is homonuclear or heteronuclear in nature. Using the energy and bonding properties of the overlapping atomic orbitals, fill molecular orbitals. Predict the molecule's properties using the diagram.
CLASSIFICATION OF PERIODIC TABLE BASED UPON BLOCK - Blogger nd 1-10 nf 1-14 (n=1,2,3,…7) SOME PROPERTIES OF MODERN PERIODIC TABLE: ... ¢ The shape of molecular orbitals depends upon the shape of combining atomic orbitals. ¢ The electrons in a molecule are present in the various molecular orbitals as the electrons of atoms ar Read more GROUP-1 and 2 - PART-A -PHYSICAL PROPERTIES - February 22, 2022 ...
› solution-answer › chapter-8Chapter 8, Problem 53GP | bartleby Give a molecular orbital description for each of the following: a. 1,3-pentadiene b. 1,4-pentadiene c. 1,3,5-he... Organic Chemistry (8th Edition) Where is transitional epithelium found and what is its importance at those sites?
4. Chemical bonding and Molecular Structure Chemical Bonding and Molecular Structu. Now one s-orbital and one p-orb hybrid orbitals overlaps with the 2p bond angle 180 0. ii) Formation of ethyne or acetylene (C In acetylene, each C atom under axially to form a C-C σ bond. The remaini bonds. Now each C atom has 2 unhybrid linear shape with bond angle 180 0.
› 48903430 › Inorganic_Chemistry_4Inorganic Chemistry 4th edition, Catherine Housecroft Enter the email address you signed up with and we'll email you a reset link.
Exploration of the Intriguing Photovoltaic Behavior for Fused ... Nonfullerene small molecular acceptors (NF-SMAs) show potential photovoltaic performance, accelerating the development of organic solar cells (OSCs). Herein, the first … Many researchers are engaged nowadays in developing efficient photovoltaic materials to accomplish the demand of modern technology.
(Get Answer) - itu Tieleionuelear Diatomic Molecules 3.15 Lithium ... The blank molecular orbital diagram shown here (Figure 1) applies to the valence of diatomic lithium, beryllium, boron, carbon, or nitrogen. ... Molecule Ground state electron configuration Bond order Number 700 Number NF (s) (02s)(()(2 Number NF( (02()2)(T2) Drag a number into each of the blank boxes above. Classify these species...
Molecular engineering of indenoindene-3-ethylrodanine ... - Nature Non-fullerene (NF) solar cells also ... Tang, S. & Zhang, J. Design of donors with broad absorption regions and suitable frontier molecular orbitals to match typical acceptors via substitution on ...
Exploration of efficient electron acceptors for organic solar cells ... Frontier molecular orbitals (FMOs) analysis. FMOs analysis helps to understand the intramolecular charge transfer (ICT) characteristics, optoelectronic properties and electron density distribution ...
PSEB 11th Class Chemistry Solutions Chapter 4 Chemical Bonding and ... In both molecules i.e., NH 3 and NF 3, the central atom (N) has a lone pair electron and there are three bond pairs. Hence, both molecules have a pyramidal shape. ... The linear combination of atomic orbitals to form molecular orbitals is possible only when they satisfied the following conditions : (i) Similar energy of combining atomic ...
› science › articlePBDB-T and its derivatives: A family of polymer donors ... May 01, 2020 · On the other hand, to obtain high V oc s in the NFA-based OSCs, the polymer donors with deep-lying HOMO levels are required. In 2017, our group reported a novel designed NFA named IT-4F by introducing fluorine on ITIC , and the lowest unoccupied molecular orbital (LUMO) level of IT-4F is much lower than ITIC.
Molecular Geometry: A Complete Overview - PSIBERG The lewis diagram of a molecule (NH3) can be drawn by following the step below; Count the total number of valence electrons in a molecule. The valence electrons of nitrogen and the fluorine atoms are added and the number comes out is 26. N + H + H + H Valence electrons = 5 + 7 + 7 + 7 = 26 Choose the central atom
Atomic orbital - Wikipedia The shapes of the first five atomic orbitals are: 1s, 2s, 2p x, 2p y, and 2p z. The two colors show the phase or sign of the wave function in each region. Each picture is domain coloring of a ψ (x, y, z) function which depend on the coordinates of one electron.
Conducting Polymer-Based Nanofibers for Advanced ... - SpringerLink According to the theory of quantum mechanics, delocalization occurs when molecular orbitals in conjugated polymer overlap with each other. In the presence of an external electric field, electrons will break away from the valence band or main chain structure, and an electric current will be formed inside the polymer, thus possessing excellent ...






















0 Response to "40 nf molecular orbital diagram"
Post a Comment