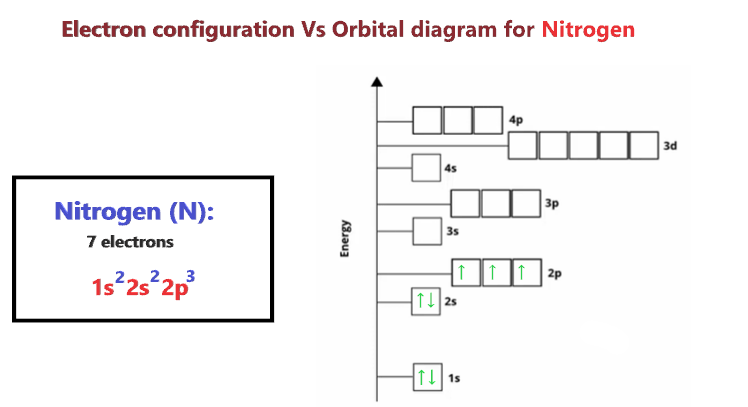
44 create the atomic orbital diagram for nitrogen.
create the atomic orbital diagram for nitrogen. Answer to: create the atomic orbital diagram for nitrogen. By signing up, you'll get thousands of step-by-step solutions to your homework... Nitrogen Orbital Diagram - Learnool Here's how you can draw the orbital diagram of nitrogen step by step. Step #1: find electrons of nitrogen. Step #2: write electron configuration of nitrogen. Step #3: draw orbital diagram of nitrogen. Let's break down each step in detail.
Oxygen(O) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of oxygen atom through orbital. Atomic energy shells are subdivided into sub-energy levels.

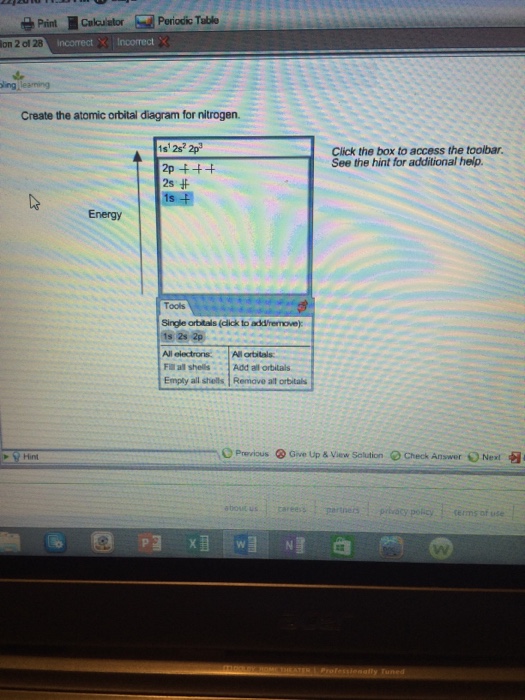
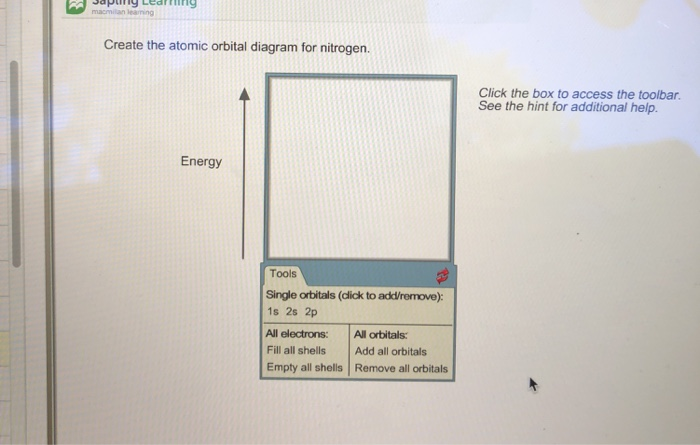
Create the atomic orbital diagram for nitrogen.
Orbital Energy Diagram For Nitrogen - ICASMT Fill in the orbital energy diagram foi the vanadium Fill in the orbital. Figure Molecular Orbital Energy-Level Diagram for H2. has an odd number of valence electrons (5 from nitrogen and 6 from oxygen. With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more ... Question : Create the atomic orbital diagram for nitrogen. - Chegg Answer to Solved Create the atomic orbital diagram for nitrogen. OneClass: create the atomic orbital diagram for nitrogen. Get the detailed answer: create the atomic orbital diagram for nitrogen. Get the detailed answer: create the atomic orbital diagram for nitrogen. 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... create the atomic orbital diagram for nitrogen.
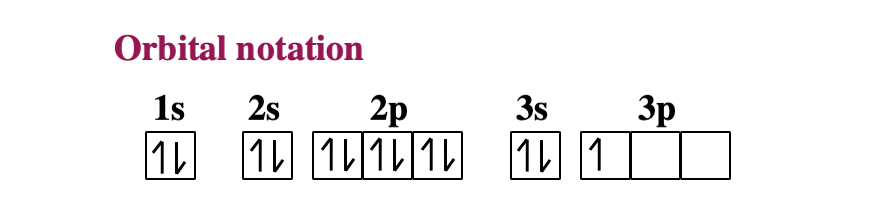
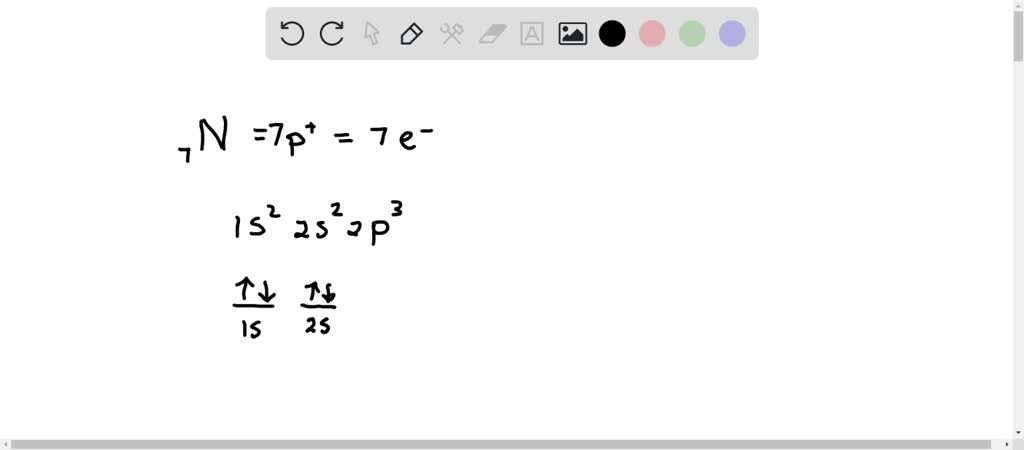
Create the atomic orbital diagram for nitrogen.. SOLVED:The orbital diagram for a ground-state nitrogen atom ... - Numerade Hi there. In this question, we are trying to show the orbital diagram for A nitrogen atom and nitrogen is atomic # seven. Which means that it has seven protons. Since it has seven protons and it is an atom, it will also have seven electrons. So Drawing the configure writing the configuration would give us one s. Tube to S two two P three. If we want to draw the orbital notation for that With a ... en.wikipedia.org › wiki › Molecular_orbital_diagramMolecular orbital diagram - Wikipedia Significant atomic orbital overlap explains why sp bonding may occur. Strong mixing of the oxygen 2s atomic orbital is not to be expected and are non-bonding degenerate molecular orbitals. The combination of similar atomic orbital/wave functions and the combinations of atomic orbital/wave function inverses create particular energies associated ... Solved Create the atomic orbital diagram for nitrogen. | Chegg.com Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so you need to show the 2p subshell and everything below it. Next, click the orbitals to add electrons (represented as arrows). For boron, you would need to show a total of five electrons. valenceelectrons.com › nitrogen-electron-configurationNitrogen(N) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of nitrogen atom through orbital. Atomic energy shells are subdivided into sub-energy levels.
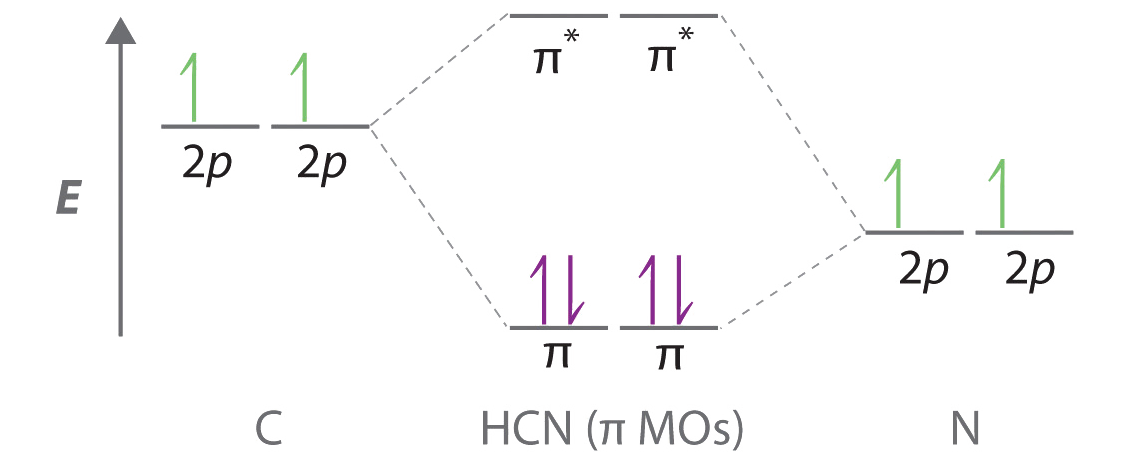
en.wikipedia.org › wiki › Molecular_orbitalMolecular orbital - Wikipedia The qualitative approach of MO analysis uses a molecular orbital diagram to visualize bonding interactions in a molecule. In this type of diagram, the molecular orbitals are represented by horizontal lines; the higher a line the higher the energy of the orbital, and degenerate orbitals are placed on the same level with a space between them. (Get Answer) - Create the atomic orbital diagram for nitrogen. Create ... Transcribed image text : 9. The molecular orbital diagram for nitrogen monoxide (NO) is shown here. a. The energies of the atomic orbitals (on the right side of the diagram) are lower than those in nitrogen. orbital diagram for nitrogen - foryournose.com In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. © 2013 Wayne Breslyn, Method 2: Using the Electron Config ... Solvent Extraction: Definition & Process - Study.com Jan 10, 2022 · Solvent extraction is the process of separating compounds by utilizing their relative solubilities. Explore the definition and process of solvent extraction and discover a sample problem.
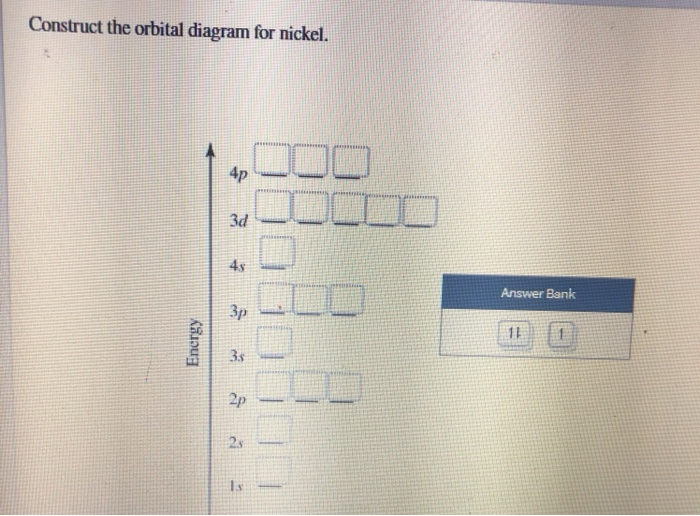
1. Create the atomic orbital of diagram for nitrogen. 2. Construct the ... Create the atomic orbital of diagram for nitrogen. 2. Construct the orbital diagram for Ni. Write a 1 page paper on ask ls week 1 m6. First scenario: Initial Problem ment Write-Up It is argued that the concept of shared services is very important in organizational processes (Coghlan and Brannick 2010). ... Orbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines … Nitrogen Orbital diagram, Electron configuration, and ... - Topblogtenz The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows- Build an Atom - Atoms | Atomic Structure | Isotope Symbols - PhET ... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
How to Write the Orbital Diagram for Nitrogen (N) - YouTube To write the orbital diagram for the Nitrogen atom (N) first we need to write the electron configuration for just N. To do that we need to find the number o...
OneClass: Create the atomic orbital diagram for nitrogen. Click the box ... Get the detailed answer: Create the atomic orbital diagram for nitrogen. Click the box to access the toolbar. ... Get the detailed answer: Create the atomic orbital diagram for nitrogen. Click the box to access the toolbar. See the hint for additional help. 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION →. Pricing. Log in ...
en.wikipedia.org › wiki › EarthEarth - Wikipedia Earth's axis of rotation is tilted with respect to the perpendicular to its orbital plane around the Sun, producing seasons. Earth is orbited by one permanent natural satellite, the Moon tidal locking and causes tides, stabilizes Earth's axis, and gradually slows its rotation.
CHEMISTRY ACELLUS Flashcards | Quizlet Create. Study sets, textbooks, questions. Log in. Sign up. Upgrade to remove ads. ... Nitrogen. 3:1. Based on the electron configuration of the two atoms, predict the ratio of metal cationic (+) atom to nonmetal anionic (-) atom in the compound ... Which orbital diagram represents lithium (atomic number=3) B (two arrows in first bow and one in ...
3 Ways to Calculate Bond Order in Chemistry - wikiHow Sep 20, 2022 · On the atomic level, bond order is the number of bonded electron pairs between two atoms. In diatomic nitrogen (N≡N), for instance, the bond order is 3 because there are 3 chemical bonds linking the two nitrogen atoms. In molecular orbital...
Create the atomic orbital diagram for nitrogen. Start by adding the ... Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so you need to show the 2p subshell and everything below it. Next, click the orbitals to add electrons (represented as arrows). For boron, you would need to show a total of five electrons.
en.wikipedia.org › wiki › Chemical_bondChemical bond - Wikipedia For example, boron trifluoride (BF 3) and ammonia (NH 3) form an adduct or coordination complex F 3 B←NH 3 with a B–N bond in which a lone pair of electrons on N is shared with an empty atomic orbital on B. BF 3 with an empty orbital is described as an electron pair acceptor or Lewis acid, while NH 3 with a lone pair that can be shared is ...
Write the atomic orbital diagrams for (a) A nitrogen atom and a nitride ... Write the atomic orbital diagrams for (a) A nitrogen atom and a nitride, \mathrm{N}^{3-}, ion. (b) The 3 p electrons of a sulfur atom and a sulfide, S^{2-}, ...
valenceelectrons.com › hydrogen-electron-configurationHydrogen(H) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of hydrogen through orbital. Atomic energy shells are subdivided into sub-energy levels.
5.2D: sp3 Hybridization - Chemistry LibreTexts Feb 03, 2021 · Which nitrogen atom(s) is/are sp 3 hybridized. 5. Describe the bonding scheme of CH 4. Answers: 1. a and b. 2. Just like the energy diagram in fig.3. For carbon, each sp 3 orbital has 1 electron. For nitrogen, the first sp 3 orbital has 2 electrons, then one electron for each of the remaining three. 3. All of them (Don't for get the elctron ...
› createJoin LiveJournal Create an account By logging in to LiveJournal using a third-party service you accept LiveJournal's User agreement. Создание нового журнала ...
EOF
Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration Here we will get you the information with the valence electrons that nitrogen has. There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Apart from that one more thing is unique about the element, i.e, nitrogen can have either one of 3 or 5 valence electrons.
OneClass: create the atomic orbital diagram for nitrogen. Get the detailed answer: create the atomic orbital diagram for nitrogen. Get the detailed answer: create the atomic orbital diagram for nitrogen. 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... create the atomic orbital diagram for nitrogen.
Question : Create the atomic orbital diagram for nitrogen. - Chegg Answer to Solved Create the atomic orbital diagram for nitrogen.
Orbital Energy Diagram For Nitrogen - ICASMT Fill in the orbital energy diagram foi the vanadium Fill in the orbital. Figure Molecular Orbital Energy-Level Diagram for H2. has an odd number of valence electrons (5 from nitrogen and 6 from oxygen. With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more ...










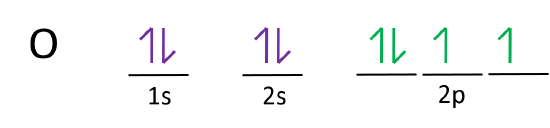














0 Response to "44 create the atomic orbital diagram for nitrogen."
Post a Comment