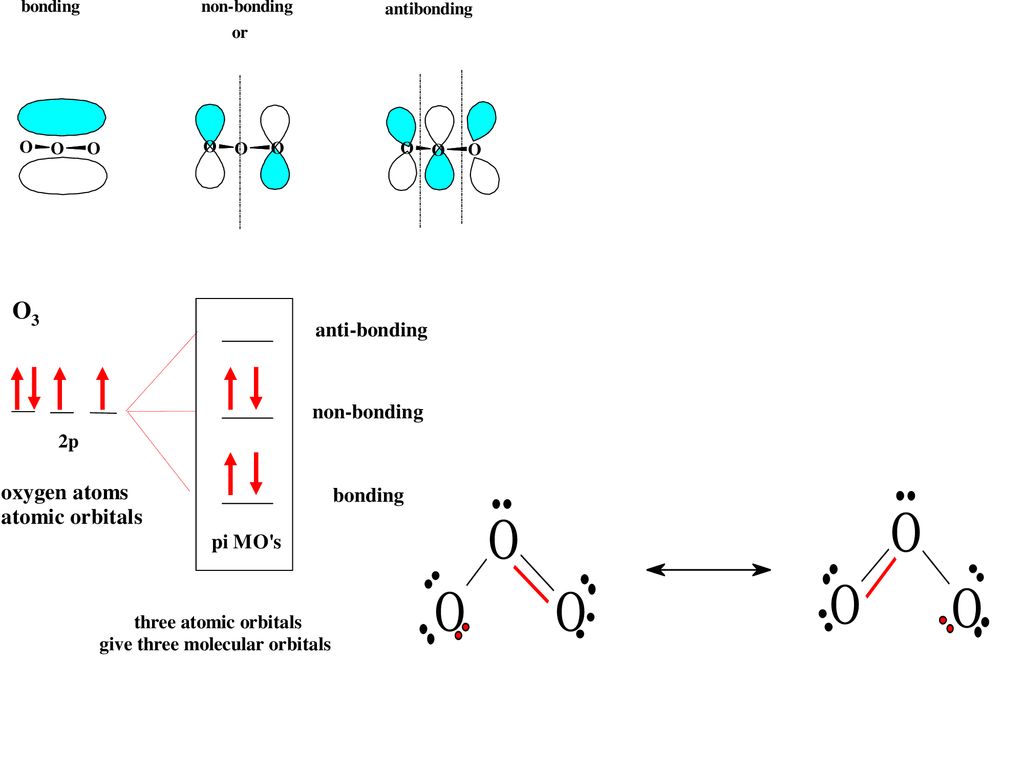
38 o3 molecular orbital diagram
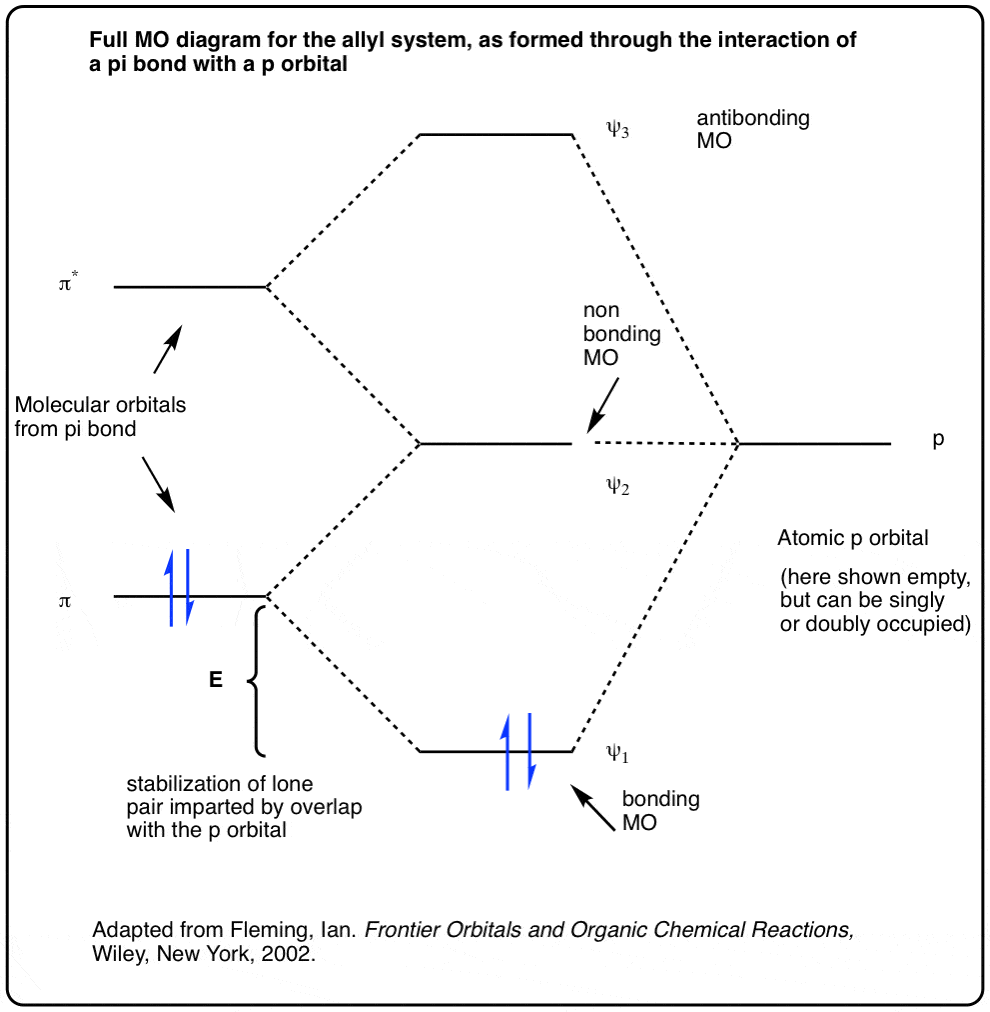
Pi Bonds over 3 Atoms The full molecular orbital diagram with the electrons is below. Nitrogen Dioxide Nitrogen dioxide has one fewer electron than ozone. Because of this, the highest energy orbital that is occupied by electrons, the 2 orbital, has only one electron. At right are the molecular orbitals for nitrogen dioxide. AB2-Molecules like OF2 and O3 - TU Braunschweig These eight orbitals harbour twelve electrons (6 of oxygen / sulfur and 3 from each flourine atom). Three bonding and two non-bonding orbitals accept a maximal number of ten electrons. For two electrons, only an antibonding orbital remains. Among the two antibonding orbitals, the 3a 1 is lower in energy and thus filled.
Synthesis, Structure, Electrochemical Mechanisms, and ... May 31, 2022 · ConspectusThe commercialization of lithium ion batteries (LIBs) triggered a new era of portable electronics and electric vehicles, which changed our daily life remarkably. Meanwhile, LIBs are promising as large-scale storage batteries in green electric grids by using renewable energy as the primary energy source. Driven by the concerns of lithium depletion and the turbulent price of Li, Ni ...

O3 molecular orbital diagram
BH3Orbitals - Yale University The following figures show how the computer parses the total electron density into SCF molecular orbitals (presented in order of increasing energy, omitting the lowest MO which is mostly the 1s core orbital on B). Since there are 7 valence-level atomic orbitals (1s on each of three H atoms plus 2s and three 2p AOs on B) there are 7 orthogonal low-energy molecular orbitals that can be made from ... Bonding in O3 and SO2 - American Chemical Society by TY Takeshita · 2015 · Cited by 30 — O3 and SO2 using both molecular orbital (MO) and valence ... represent the GVB wave function using simple orbital diagrams. Ozone | O3 - PubChem Ozone | O3 | CID 24823 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem ...
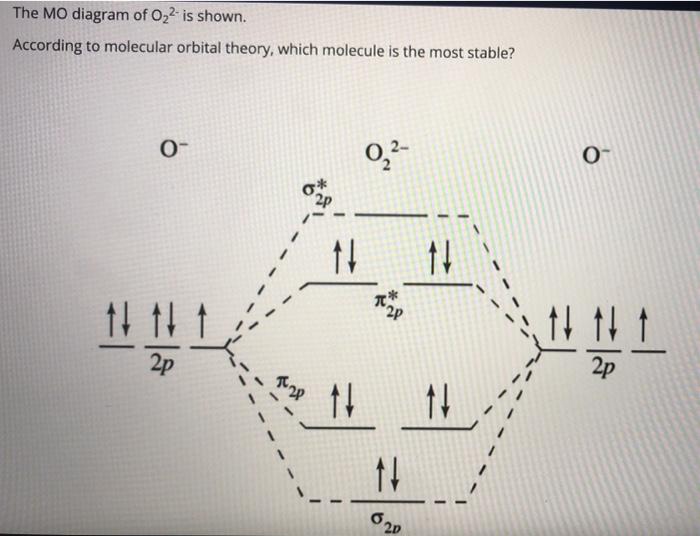
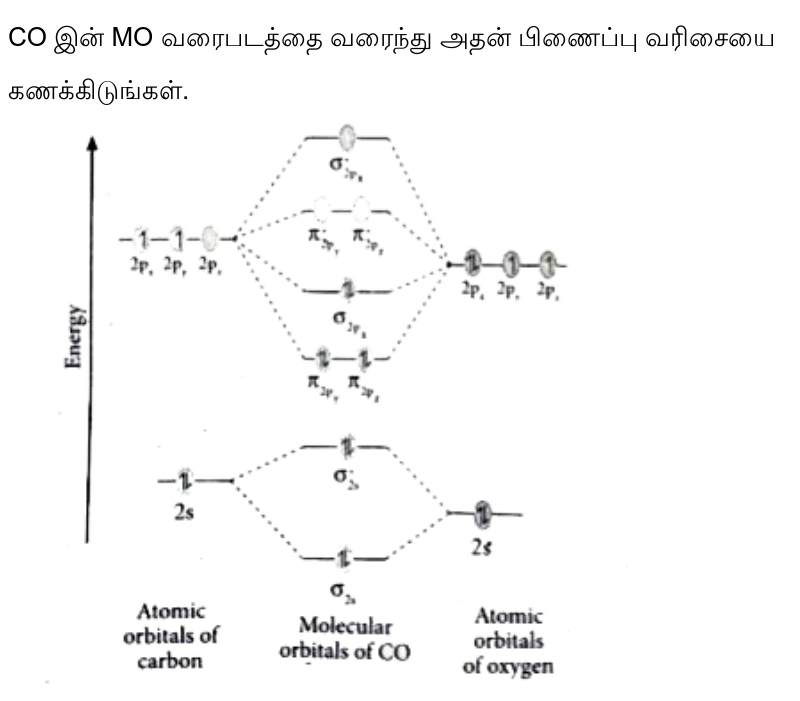
O3 molecular orbital diagram. Molecular orbital diagram of TiO2, Ti2O3, TiO ... - ResearchGate According to crystal field splitting (Figure 9), Ti (3d, 4s, 4p) orbitals are linearly coalesced with O (2s, 2p) orbitals to form molecular orbitals as depicted in Figure 9 The defects and non ... Chemistry: Atoms First 3rd Edition Textbook Solutions | bartleby Chapter 3 - Quantum Theory And The Electronic Structure Of Atoms Chapter 3.1 - Energy And Energy Changes Chapter 3.2 - The Nature Of Light Chapter 3.3 - Quantum Theory Chapter 3.4 - Bohr's Theory Of The Hydrogen Atom Chapter 3.5 - Wave Properties Of Matter Chapter 3.6 - Quantum Mechanics Chapter 3.7 - Quantum Numbers Chapter 3.8 - Atomic Orbitals Chapter 3.9 - Electronic Configurations Chapter ... PDF Chemistry 2000 Slide Set 5: Molecular orbitals for polyatomic molecules Molecular orbitals for polyatomic molecules Marc R. Roussel January 8, 2020 Marc R. Roussel MOs for polyatomic molecules January 8, 20201/23. LCAO-MO theory for polyatomic molecules LCAO-MO theory can of course be used for molecules of any size. This becomes di cult to do qualitatively with many nuclei or complex Schupf Computational Chemistry Lab - Colby College Molecular Orbital Energies. The orbital energies are given in eV, where 1 eV=96.49 kJ/mol. Orbitals with very low energy are core 1s orbitals. More antibonding orbitals than you might expect are sometimes listed, because d orbitals are always included for heavy atoms and p orbitals are included for H atoms.
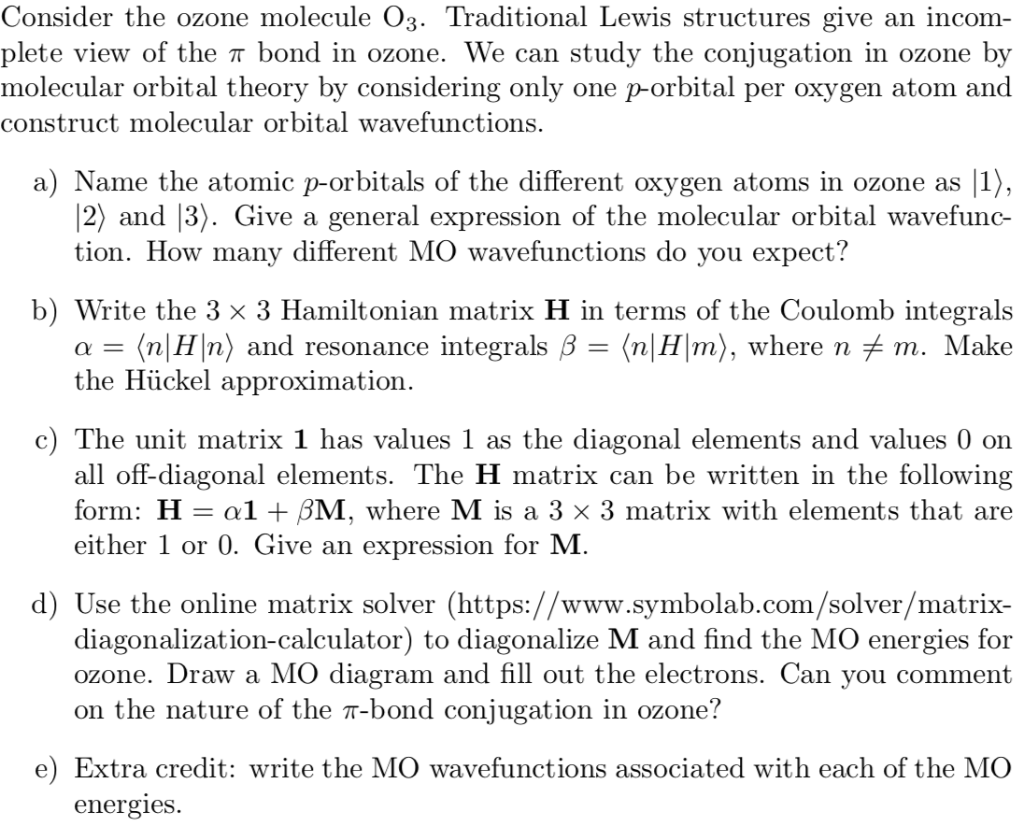
Molecular Orbitals for Ozone Purpose - Colby College Make sure you understand how the molecular orbital coefficients are determined and their meaning. Draw similar diagrams for other orbitals in the print out.10 pages O3 Lewis Structure | Step By Step Drawing - What's Insight The Lewis diagram of O3 shows three oxygenatoms having eighteen dots, of valence electrons. Where six are arranged, around each oxygen atom. Step-3: Place remaining electrons around the other atoms The lateral oxygen atoms have achieved octet as they have eight electrons surrounding them. But, the central atom only has six electrons around it. A molecular orbital diagram of ozone is at right; but its not ... Recall that bonding is a valence-electron phenomenon, that only valence orbitals contribute to a molecular orbital diagram, and that electrons generally fill ... Ozone Structure and Molecular Orbitals - Massachusetts Institute of ... Ozone Structure and Molecular Orbitals. Right-click in the Jmol window to bring up the menu, and choose "console" to bring up the console window. Type "mo homo" in the console to view the HOMO of the molecule and find out what its number is, or "mo lumo" to view the LUMO. Orbitals are numbered in ascending energy, and any MO can be selected by ...
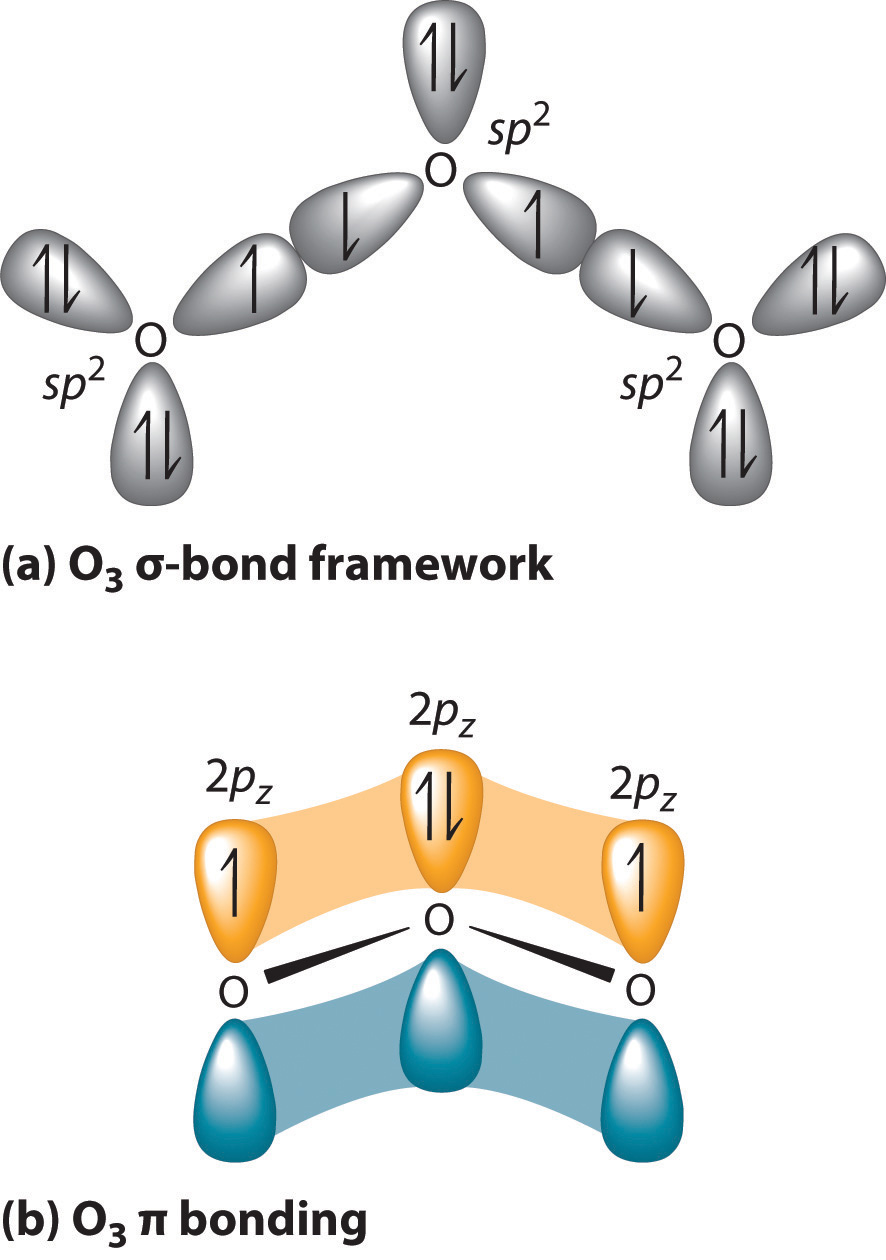
Hybridization of central atom in O3 - CHEMISTRY COMMUNITY In the Fall 2012 quiz for preparation for quiz 2 in the workbook, #2 asks for the hybridization of the central atom in O3. The answer is sp2 and I am confused as to how the central atom, which would be O, could have an sp2 hybridization. After drawing the Lewis Structure, I know there needs to 18 electrons (3x6 electrons) and knowing that, it ... How is the molecular geometry of O3 determined? - Quora The molecular geometry of o3 is trigonal planner.It is due to the presence of lone pair on the central metal atom which repels the electrons in the two bonds, causing the atom to adopt a bent molecular geometry.. Promoted by The Penny Hoarder Kyle Taylor Founder at The Penny Hoarder (2010-present) Updated Aug 4 Dr. Sharad Pratap SiNgh (SPS) Why can't the O3 structure be this? (see image) : r/chemhelp - reddit Oxygen has 6 electrons in its outer most shell. In order to be more energetically stable it shares 2 electrons with the other oxygen atom to acquire 8 electrons in its valence shell. With O3 now one Oxygen atom uses a pair of non-bonded electrons to form a coordinate bond with the extra O atom to form O3. It kind of is but it isn't exactly. O3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram ... O3 Molecular Orbital Diagram (MO) The molecular orbital theory is one of the major revolutionary concepts of chemical bonding. It uses quantum mechanics to give us a detailed almost explanatory diagram of the bonding nature inside a molecule. Here is a diagrammatic representation of the MO diagram of ozone. Ozone is a trigonal planar molecule.
Answered: Calculate the enthalpy change for this… | bartleby Sep 14, 2022 · A: Solution: Here we know, For principal number 4 indicates 4 th shell and l =2 corresponding orbital… question_answer Q: A solution is prepared by dissolving 26.42 g glycerin (C3H803, molar mass = 92 g/mol) in water.
Centaur (small Solar System body) - Wikipedia In planetary astronomy, a centaur is a small Solar System body with either a perihelion or a semi-major axis between those of the outer planets (between Jupiter and Neptune). ). Centaurs generally have unstable orbits because they cross or have crossed the orbits of one or more of the giant planets; almost all their orbits have dynamic lifetimes of only a few million years, but there is one ...
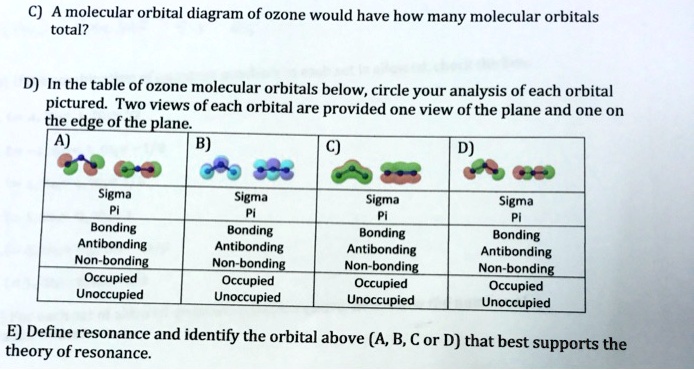
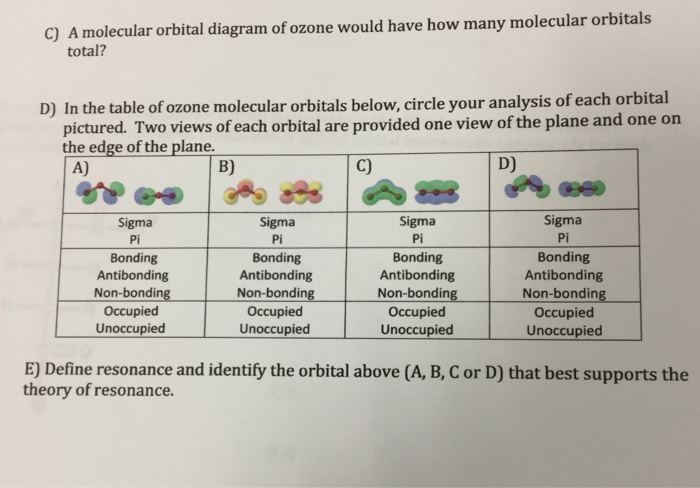
Solved 4) The Lewis structure for ozone (O3) and the - Chegg There are four total electrons in the shared T-system of ozone. The highest energy electrons are paired in molecular orbital (A/B/C). e. Based on molecular orbital diagram for ozone, the highest energy electrons in the -system are shared by the oxygen atoms labeled (1/2/3, choose all that apply). Previous question Next question
FIG. 1. Schematic molecular orbital diagram of O 3 and O ϩ 3... Schematic molecular orbital diagram of O 3 and O ϩ 3 illustrating the Source publication +4 High-resolution pulsed-field-ionization zero-kinetic-energy photoelectron spectroscopic study of the two...
O3 Lewis Structure, Polarity, Hybridization, Shape and Molecular Geometry O3 Molecular Geometry As the hybridization of the molecule determines its shape, we can now know the molecular geometry of Ozone. Ozone has sp2 hybridization means that it should have a trigonal planar shape. But as the structure of Ozone has resonance and one lone pair of electrons, the angle between the molecules is less than 120 degrees.
Radical (chemistry) - Wikipedia Delocalization effects can also be understood using molecular orbital theory as a lens, more specifically, by examining the intramolecular interaction of the unpaired electron with a donating group’s pair of electrons or the empty π* orbital of an electron-withdrawing group in the form of a molecular orbital diagram. The HOMO of a radical is ...
Inorganic Chemistry 4th edition, Catherine Housecroft Purple acid phosphatases (PAPs) are a group of metallohydrolases that contain a dinuclear Fe(III)M(II) center (M(II) = Fe, Mn, Zn) in the active site and are able to catalyze the hydrolysis of a variety of phosphoric acid esters.
Solved 4) The Lewis structure for ozone (O3) and the | Chegg.com In ozone, each atom donates shared 7-system. (enter a numeral) p-orbital(s) to the b. Molecular orbital (A/B/C) contains the fewest nodes and is the (highest/lowest) energy re-type; Question: 4) The Lewis structure for ozone (O3) and the possible r-type molecular orbitals for a three-atom molecule are shown below. Answer the following questions ...
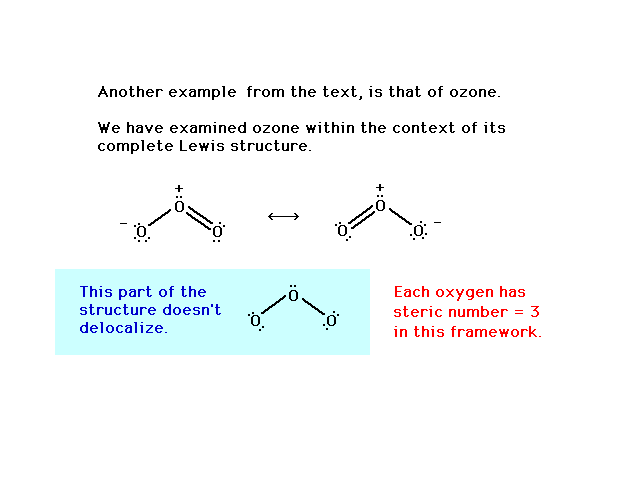
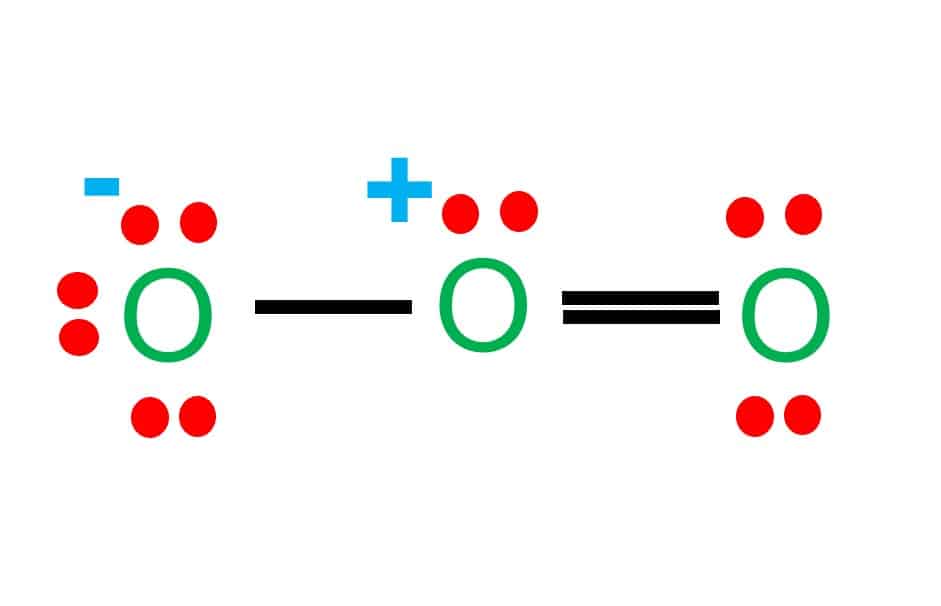
Resonance Structures of O3, Ozone - YouTube Ozone molecules are three oxygen atoms bonded in succession, they are NOT in a ring. The middle oxygen, according to the Lewis Structure, needs one double bond and one single bond ... but because...
O3 - ozone Animated Molecular Orbitals; Spectroscopy. Introduction; Symbols, Terminology and Constants; Orbitals; Vibrational spectroscopy. Water; Hydrogen Chloride; Carbon Dioxide; ... Home / Gallery / O3 - ozone. O 3 - ozone. CONTROLS . 5 (1) How useful was this page? Click on a star to rate it! Submit Rating . Average rating 5 / 5. Vote count: 1.
O3 Lewis Structure - Ozone - YouTube This chemistry video tutorial explains how to draw the lewis structure of O3. It also discusses the molecular geometry, bond angle, hybridization, and forma...
SO3 Lewis Structure, Molecular Geometry, and Hybridization The hybridization of SO3 is sp2. It is determined with the help of formula: Number of hybrid orbitals = Number of sigma bonds + Number of lone pairs. In a single shared double covalent bond, there exists one sigma (σ) bond and one pi (π) bond. So, the total number of sigma bonds in a single SO3 molecule is three, and the total number of lone ...
4.11: Multiple Bonds in MO Theory - Chemistry LibreTexts 8 May 2021 — Each oxygen atom in ozone has 6 valence electrons, so O3 has a total of 18 valence electrons. Subtracting 14 electrons from the total gives us 4 ...
band structure - Predict the density of states (DOS) diagram for ... The idea is that once the molecular orbital diagram is drawn, it predicts the shape of the density of states by means of the type of orbital interaction (sigma, pi, delta): The theory on which the problem is based is shown in this slide, in case I have not explained it well. band-structure from-chem-se orbitals perovskites Share
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM - University of California, Irvine Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy molecular orbital with respect to the 3s orbital of sulfur. This lowest energy orbital is essentially nonbonding.
O3 resonance structures [with diagrams] - ace organic chem 2 bonds + 2 lone pairs = neutral oxygen atom 1 bond + 3 lone pairs = negatively charged oxygen atom 3 bonds and 1 lone pair = positively charged oxygen So, in summary, if a professors asks you how many resonance structures can be drawn for ozone O 3, your answer should be a (confident) two. Reference: Ozone and the ozonolysis of alkenes
O3 Lewis structure, Molecular geometry, Bond angle, Shape Ozone (O 3) molecule has a bent or V-shape and molecular geometry. The ideal electron geometry of O 3 is trigonal planar. The Lewis dot structure of O 3 has two equivalent resonance forms. There are +1 and -1 formal charges present on two of the three bonded oxygen atoms in the O 3 Lewis structure.
H3 Molecular Orbital Diagram H3+ molecular orbital diagram H3: From linear to bent What happens if we begin with H3.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
Which d orbitals of sulfur take part in the pi bonds of SO3? $\begingroup$ If your textbook still needs to use d orbitals to explain the hypercoordinate molecules, then you should get a new one. For the complete molecular orbitals of $\ce{SO3}$ have a look at this question and answer.Apart from this the question is incomplete. In order to determine which orbitals take part in bonding, you have to know the position of the symmetry defining elements.
What is the differences between Kollman and Gasteiger charges? **Molecular mechanical models for organic and biological systems going beyond the atom centered two body additive approximation. Cieplak P, Caldwell J, Kollman P. J Comput Chem.2001 Jul 30;22(10 ...
Ozone Molecular Orbital Diagram Our MO treatment of ozone is entirely analogous to the 4-electron H3- anion. We map that solution. Figure Molecular Orbital Energy-Level Diagram for \ (\pi\) Each oxygen atom in ozone has 6 valence electrons, so O 3 has a total of Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation.
O3 Molecular Orbital Diagram - schematron.org O3 Molecular Orbital Diagram 08.10.2018 5 Comments The ozone molecule's Lewis structure shows that even the preferred structure The pi molecular orbital energy diagram for ozone into which are distributed four . Please draw MO diagram for ozone (O3). I saw a pic for O3 where the 2s of O was interacting with both bonding and antibonding.
Slides24 - andrew.cmu.ed
Ozone | O3 - PubChem Ozone | O3 | CID 24823 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem ...
Bonding in O3 and SO2 - American Chemical Society by TY Takeshita · 2015 · Cited by 30 — O3 and SO2 using both molecular orbital (MO) and valence ... represent the GVB wave function using simple orbital diagrams.
BH3Orbitals - Yale University The following figures show how the computer parses the total electron density into SCF molecular orbitals (presented in order of increasing energy, omitting the lowest MO which is mostly the 1s core orbital on B). Since there are 7 valence-level atomic orbitals (1s on each of three H atoms plus 2s and three 2p AOs on B) there are 7 orthogonal low-energy molecular orbitals that can be made from ...












0 Response to "38 o3 molecular orbital diagram"
Post a Comment