41 pf5 molecular orbital diagram
What is the hybridization of PF5? | Socratic In P F 5 , the valence electrons of phosphorus are 5. and we take the oxidation state of halogens as 1. So, for one F , electron is 1 and for F 5 the no. of electrons is 5. therefore, (from phosphorus and fluorine) 5-5=0 electrons = 0 lone pairs. there are 5 sigma bonds in this compound. So, 5 sigma bonds + 0 lone pairs = 5. → sp3d Answered: Phosphorus pentafluoride, PF5, is a… | bartleby Science Chemistry Q&A Library Phosphorus pentafluoride, PF5, is a colourless gas, and it is used as a source of phosphorus in semi-conductors and as a catalyst in ionic polymerization. Use VSEPR theory to predict the molecular geometry of PF 5. Phosphorus pentafluoride, PF5, is a colourless gas, and it is used as a source of phosphorus in semi ...
PF5 Molecular Geometry / Shape and Bond Angles - YouTube A quick explanation of the molecular geometry of PF5 including a description of the PF5 bond angles. Looking at the PF5 Lewis structure we can see that there are five Fluorine (F) atoms attached to...

Pf5 molecular orbital diagram
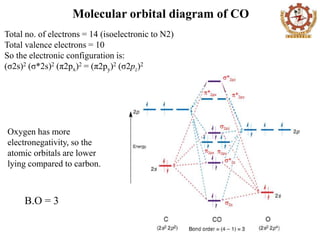
CH2O lewis structure, molecular geometry, bond angle, hybridization? Formaldehyde (CH2O) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Formaldehyde is an organic compound that appears as a colorless gas with the chemical formula CH2O. It is the simplest aldehyde made up of two hydrogens, one carbon, and one oxygen. It is widely used as a preservative because of its antibacterial ... PCl5 Lewis Structure, Molecular Geometry ... - Techiescientist Molecular Orbital Theory and MO diagram of PCl5 Molecular Orbital theory makes use of Molecular Orbital diagrams to showcase a clear picture of the state of electrons in an atom. While the Valence Bond theory and VSPER give an idea of an atom's properties, it is not useful in the case of certain molecules. Answered: briefly explain how your molecular… | bartleby Science Chemistry Q&A Library briefly explain how your molecular orbital diagram demonstrate the hypervalency of PF5 comment upon the bond order of PF5 The D3h molecules , PF5 is commonly referred to as hypervalent molecular species
Pf5 molecular orbital diagram. On the basis of hybridisation, explain the shape of phosphorus penta ... In PF5, the central atom is 15P. The electronic configurations of phosphorus and fluorine atoms are as follows:It is the case of sp3d hybridization in which one 3s, three 3p and one 3d orbital of P-atom having nearly same energy intermix to given five sp3 dihybrid orbitals. Now five hybrid orbitals are available for a combination which permits the formation of five covalent bonds with five ... LiveInternet @ Статистика и дневники, почта и поиск Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu. BrF5 Lewis Structure, Molecular Structure, Hybridization, Bond Angle ... This gives the molecule a Square Pyramidal shape. Therefore, BrF5 has a square pyramidal molecular geometry and an Octahedral electronic shape. Concluding Remarks Let's quickly summarize the salient features of Bromine Pentafluoride BrF5 comprises a Bromine atom surrounded by five Fluorine atoms. PF5 Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair And ... PF5 has a molecular weight equal to 125.96 g/mol. In appearance, it is observed to be a gas which is colorless and has one so pleasant odor. It's observed density is around 5.527 kg/m3. Its observed melting point is -93.78 degrees Celsius and boils at a temperature of -84.6 degrees Celsius.
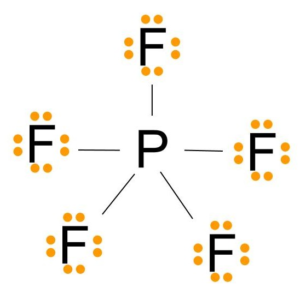
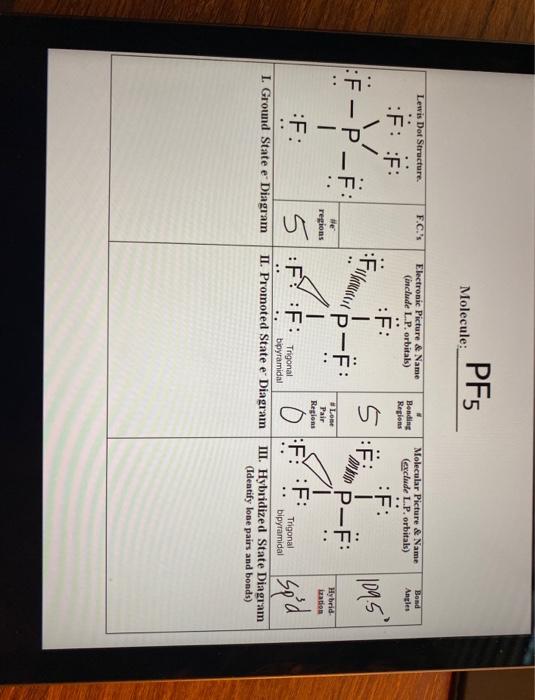
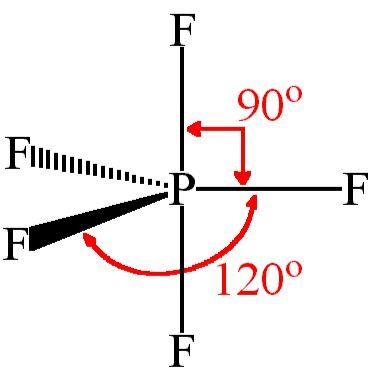
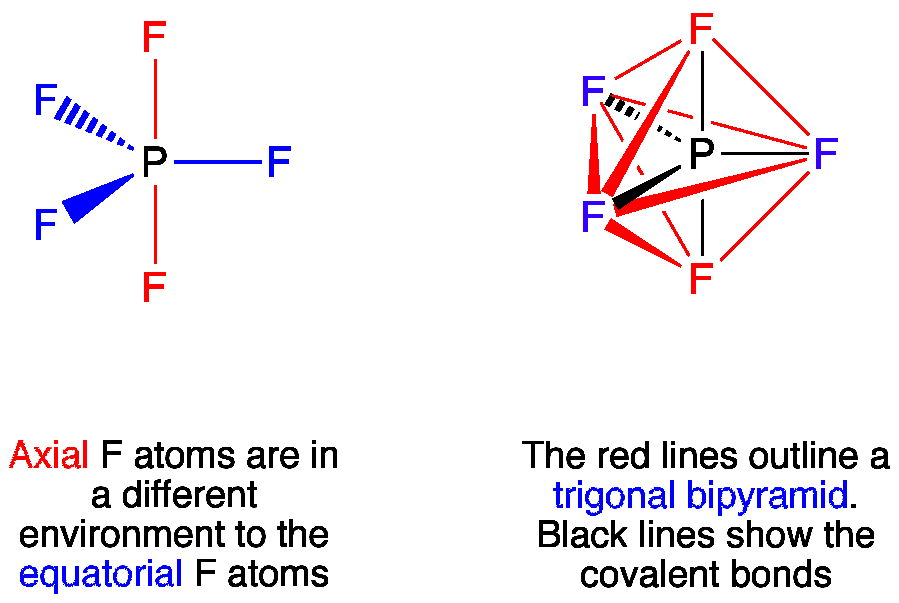
VSEPR PF5 Phosphorus Pentafluoride - ChemTube3D PF5 Phosphorus Pentafluoride View Live Phosphorus pentafluoride has 5 regions of electron density around the central phosphorus atom (5 bonds, no lone pairs). The resulting shape is a trigonal bipyramidal in which three fluorine atoms occupy equatorial and two occupy axial positions. What is the molecular geometry of the SF5+ ion? - AnswersToAll With this model we can draw a series of resonance structures as shown below for PF5. Thus, PF5 has net four covalent bonds and one ionic bond. What is the shape of SF4? Trigonal bipyramidal (sp3d) is the shape of SF4 with one equatorial position occupied by 1 lone pair. It has a see-saw shape as it contains four bond pairs and one lone pair. ACS Applied Materials & Interfaces | Ahead of Print Articles ASAP (as soon as publishable) are posted online and available to view immediately after technical editing, formatting for publication, and author proofing. The structure of PF5 molecule is A Square planar B class 11 ... - Vedantu Stericnumber = 5 + 5 2 = 5 S t e r i c n u m b e r = 5 + 5 2 = 5. The steric number of PF5 P F 5 is five. The structure of PF5 P F 5 is, In PF5 P F 5 the central atom is phosphorus has five bonding domains. The hybridization of PCl5 P C l 5 is sp3d s p 3 d. The molecular geometry is trigonal bipyramidal. The sp3d s p 3 d hybridization:
What is the structure of PF5, and how can we explain its geometry? Originally Answered: What is PF5's molecular geometry? Phosphorus is in group 15 and has 5 valence electrons. Fluorine is in group 17 and has 7 valence electrons. The 5 electrons on P would bond to F, resulting in an expanded octet on phosphorus. Phosphorus would have 10 electrons around it, dividing this by 2 gives 5 electron pairs. Hybridization of BrF5 - Hybridization of Br (Bromine) in BrF5 - BYJUS In BrF 5, one 4s, three 4p and two 4d orbitals take part in hybridization. The central atom bromine forms 5 sigma bonds with fluorine atoms. Lone pairs are found in one of the hybrid orbitals. BrF 5 Molecular Geometry And Bond Angles. BrF 5 molecular geometry is said to be square pyramidal with a bond angle of 90 o each. The structure of PF5 molecule is - Toppr Ask Answer verified by Toppr. Was this answer helpful? Column-I and Column-II contains four entries each. Entries of Column-I are to be matched with one or more than one entries of Column-II. Each entry of Column-I may have the matching with one or more than one entries of Column-II. Solved 2. In this question, you will examine the structure - Chegg (a) Draw a Lewis dot structure for PF5, including all necessary resonance contributors (keeping the octet rule in mind). Indicate the molecular geometry, and assign the correct point group. (b) How do the valence orbitals (3S, 3px, 3py, 3pz) on the central P atom transform under this point group?
ICl5 Molecular Geometry, Bond Angles (and Electron Geometry An explanation of the molecular geometry for the ICl5 (Iodine pentafluoride) including a description of the ICl5 bond angles. The electron geometry for the I...
Is PF5 Polar Or Nonpolar | All About PF5 Polarity & Shape - Gotryus The molecular geometry of PF5 is triangular bipyramidal. With the same and different plane at a different bond angle. The (X-Y) plane make the bond angle of 120 degrees and z-axis make the bond of 90 degrees. To draw PF5 Lewis structure, watch the above video or follow the given instructions.
Solved Determine the point group of the PF5 molecule (use - Chegg Expert Answer. 100% (7 ratings) Outermost electronic configuration of P is 3s2 3p3 Number of valence electrons = 5 Number of electrons from five F = 5 Total number of electrons = 10 Number of bond pair …. View the full answer. Previous question Next question.
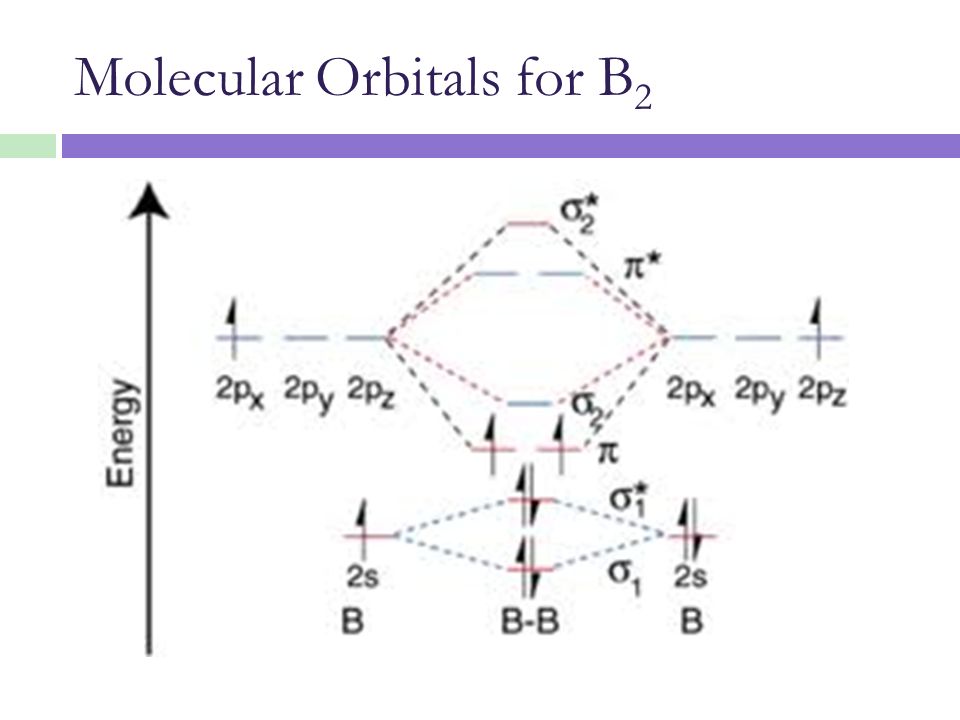
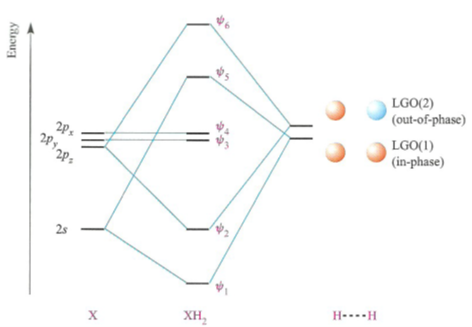
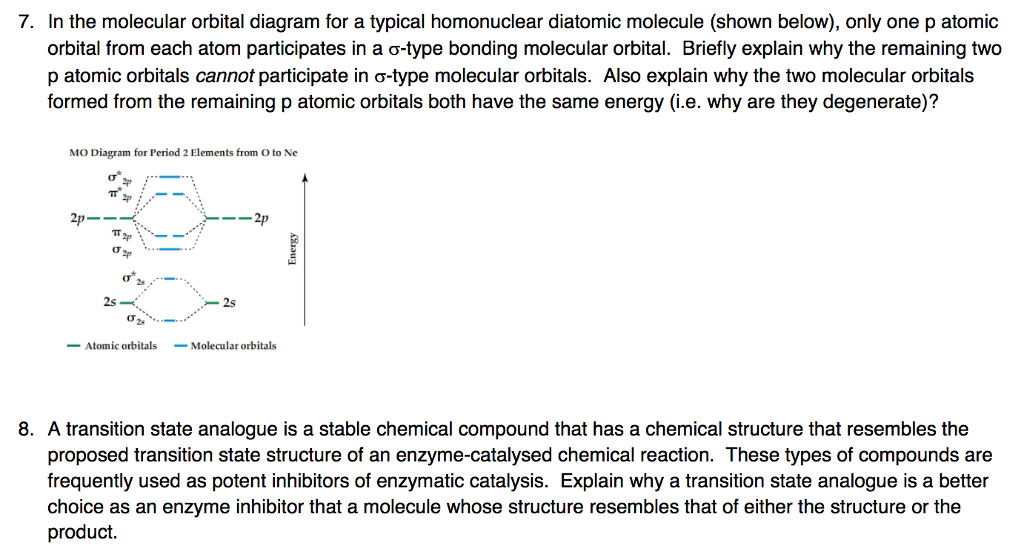
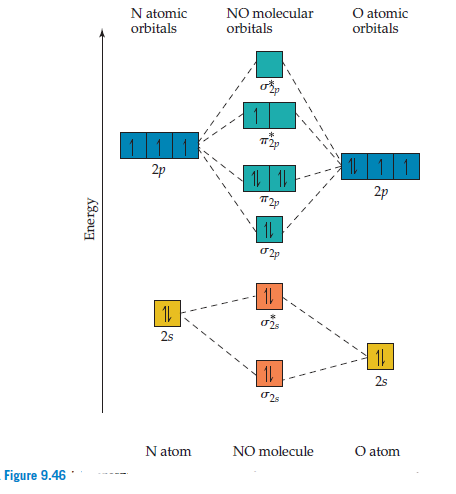
PDF Polyatomic Molecular Orbital Theory - La Salle University MO diagram of homonuclear diatomic molecules • Filling the resulting MO's of homonuclear diatomic molecules with electrons leads to the following results: Bond order = ½ (#Bonding e's - #Antibonding e's) A B 1σg 2σu 1s 1s 2s 2s 2p 2p 3σg 4σu 1πu 5σg 2πg 6σu
Inorganic Chemistry 4th edition, Catherine Housecroft Purple acid phosphatases (PAPs) are a group of metallohydrolases that contain a dinuclear Fe(III)M(II) center (M(II) = Fe, Mn, Zn) in the active site and are able to catalyze the hydrolysis of a variety of phosphoric acid esters.
IF5 Molecular Geometry - Science Education and Tutorials Iodine and fluorine atoms have s,p, and d orbitals. The sp3d2 hybridization of the IF5 molecule is formed when one S orbital, three p orbitals, and two d orbitals join together to form the IF5 molecular orbital. Molecular Geometry Notation for IF5 Molecule : Determine the form of IF5 molecular geometry using VSEPR theory.
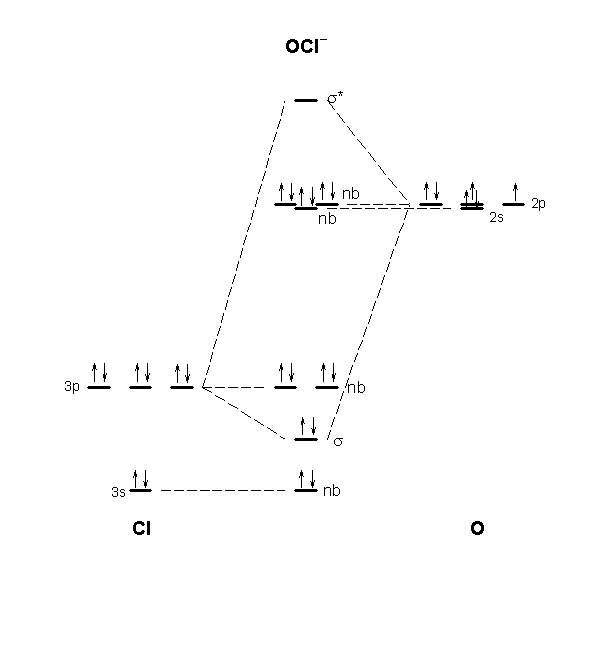
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM - University of California, Irvine orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the infinite-fold rotation axis of the orbitals on the basis of the change in wave function sign upon crossing the nodes on the bond axis. 5.10 a. OF- has 14 valence electrons, four in the π 2p* orbitals (see the diagram in the answer to Problem 5.9). b.
PF5 lewis structure, molecular geometry, bond angle, hybridization PF 5 lewis structure is made up of five P-F bonds, with a phosphorus (P) atom in a central position and all five fluorine (F) as outer atoms in the lewis diagram. The lewis structure of PF 5 contains a total of 5 bond pairs and 15 lone pairs (3 lone pairs on each fluorine atom). The drawing of the PF 5 lewis's structure is very easy and simple.
Clbr3 molecular geometry | 💖PPT - Chapter 9: Molecular Structures ... Pf5 molecular geometry. slideplayer.com Molecular Geometry and Hybrid Orbitals - ppt video online do. slideplayer.com CHEMICAL BONDING Chapter ppt download. youtube.com Polarity and Molecular Geometry - YouTube. slideplayer.com Unit 2 (Chp. 8,9): Bonding & Molecular Geometry - ppt video .
Electron Diffraction Study of the Structure of PF5 Crystal and molecular structure of 2,2,3,3-tetramethyl-5-dimethylamino-7,8-diphenyl-1,4,6,9-tetraoxa-5-phospha (PV)spiro [4.4]non-7-ene, (C6H12O2) (C14H10O2)PN (CH3)2. Inorganic Chemistry 1977, 16 (9) , 2299-2305. D. J. Brauer, H. Buerger, and G. Pawelke.
PF5 Lewis structure, Molecular Geometry, Bond angle and Shape PF5 Hybridization The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3, but when it is in an excited state, the electrons from 3s orbital get unpaired. There are five half-filled orbitals: one s orbital, three p orbitals, and one d orbital.
What is the geometry of:(i) PF5 molecule(ii) SF6 molecule? from ... The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. Each sp 1 hybrid orbital has s-character and The molecular orbital structure of ethylene: In ethene molecule, each carbon atom undergoes sp 2 hybridisation.
PBr5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity PBr5 or Phosphorous Pentabromide is a compound that consists of 5 molecules of Bromine and 1 molecule of Phosphorus. It appears to be a yellow crystalline solid. The structure of PBr5 in the solid-state is PBr4+ Br− whereas in the vapor phase it dissociates to become PBr3Br2.
Answered: briefly explain how your molecular… | bartleby Science Chemistry Q&A Library briefly explain how your molecular orbital diagram demonstrate the hypervalency of PF5 comment upon the bond order of PF5 The D3h molecules , PF5 is commonly referred to as hypervalent molecular species
PCl5 Lewis Structure, Molecular Geometry ... - Techiescientist Molecular Orbital Theory and MO diagram of PCl5 Molecular Orbital theory makes use of Molecular Orbital diagrams to showcase a clear picture of the state of electrons in an atom. While the Valence Bond theory and VSPER give an idea of an atom's properties, it is not useful in the case of certain molecules.
CH2O lewis structure, molecular geometry, bond angle, hybridization? Formaldehyde (CH2O) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Formaldehyde is an organic compound that appears as a colorless gas with the chemical formula CH2O. It is the simplest aldehyde made up of two hydrogens, one carbon, and one oxygen. It is widely used as a preservative because of its antibacterial ...



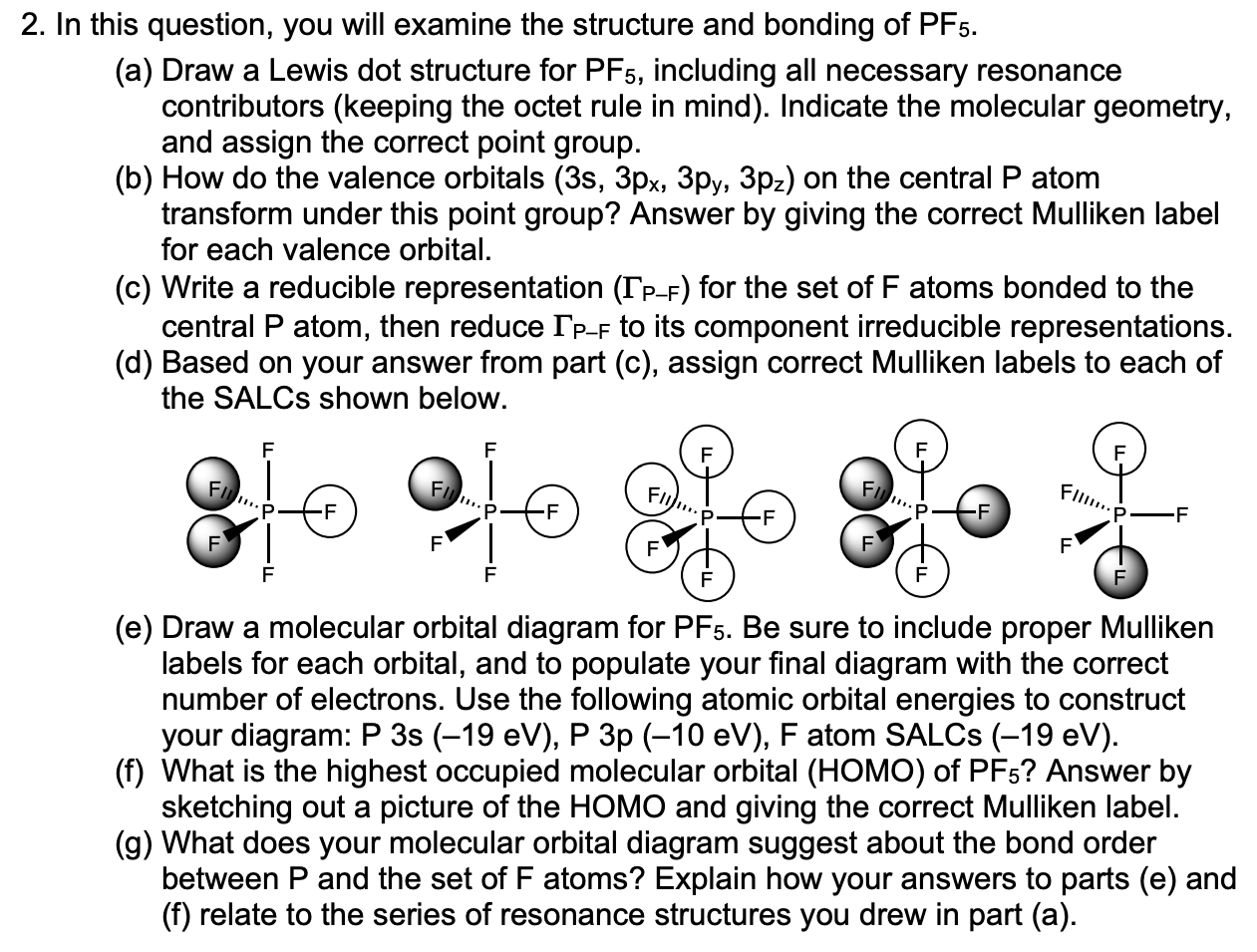

![PDF] Molecular orbital theory of pentacoordinate phosphorus ...](https://d3i71xaburhd42.cloudfront.net/93aff2f49ff8d7ba44efd0e475aff597fadad204/3-Figure2-1.png)







0 Response to "41 pf5 molecular orbital diagram"
Post a Comment