40 xef4 molecular orbital diagram
Molecular Orbital Diagram Maker Molecular Orbital Diagram Maker. ©2022 Prof Adam J Bridgeman | close window. What is the hybridization of XeF4? - Quora Answer (1 of 12): In XeF4, central atom Xe is sp3d2 hybridised having 2 lone pair on it so shape of molecule will be square planer. You can calculate its hybridisation using simple merhod, (1) Steric number rule, generally used in organic molecule to calculate hybridisation . Hybrid orbitals = ...
sciedutut.com › scl2-molecular-geometrySCl2 Molecular Geometry - Science Education and Tutorials Key Points To Consider When drawing The SCl2 Molecular Geometry. A three-step approach for drawing the SCl2 molecular can be used. The first step is to sketch the molecular geometry of the SCl2 molecule, to calculate the lone pairs of the electron in the central sulfur atom; the second step is to calculate the SCl2 hybridization, and the third step is to give perfect notation for the SCl2 ...
Xef4 molecular orbital diagram
Is XeF4 Polar or Nonpolar? All You Need to Know XeF4 is nonpolar. Using the Lewis Structure, we can identify the molecular geometry of XeF4. The lone pairs of electrons and the bond angles of all the atoms created nonpolar molecules in noble gases like Xe. The F-Xe-F bond angle is 90 degrees, and it creates a square planar shape. Is XeF4 Polar or Nonpolar? 2021 Beginner's Guide XeF4 Molecular Shape Xenon Tetrafluoride is a combination of noble gas Xe and F atoms and, you can draw the Lewis Structure to determine its physical structure. The XeF4 has a total of 36 valence electrons, and because the central Xenon atom with twelve atoms has two lone pairs. It will help with the repulsion and will create a perpendicular plane. Projection operator method: sigma molecular orbitals of ... Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.00:15 Structure of xenon tetrafluoride03:08 Reducible representation...
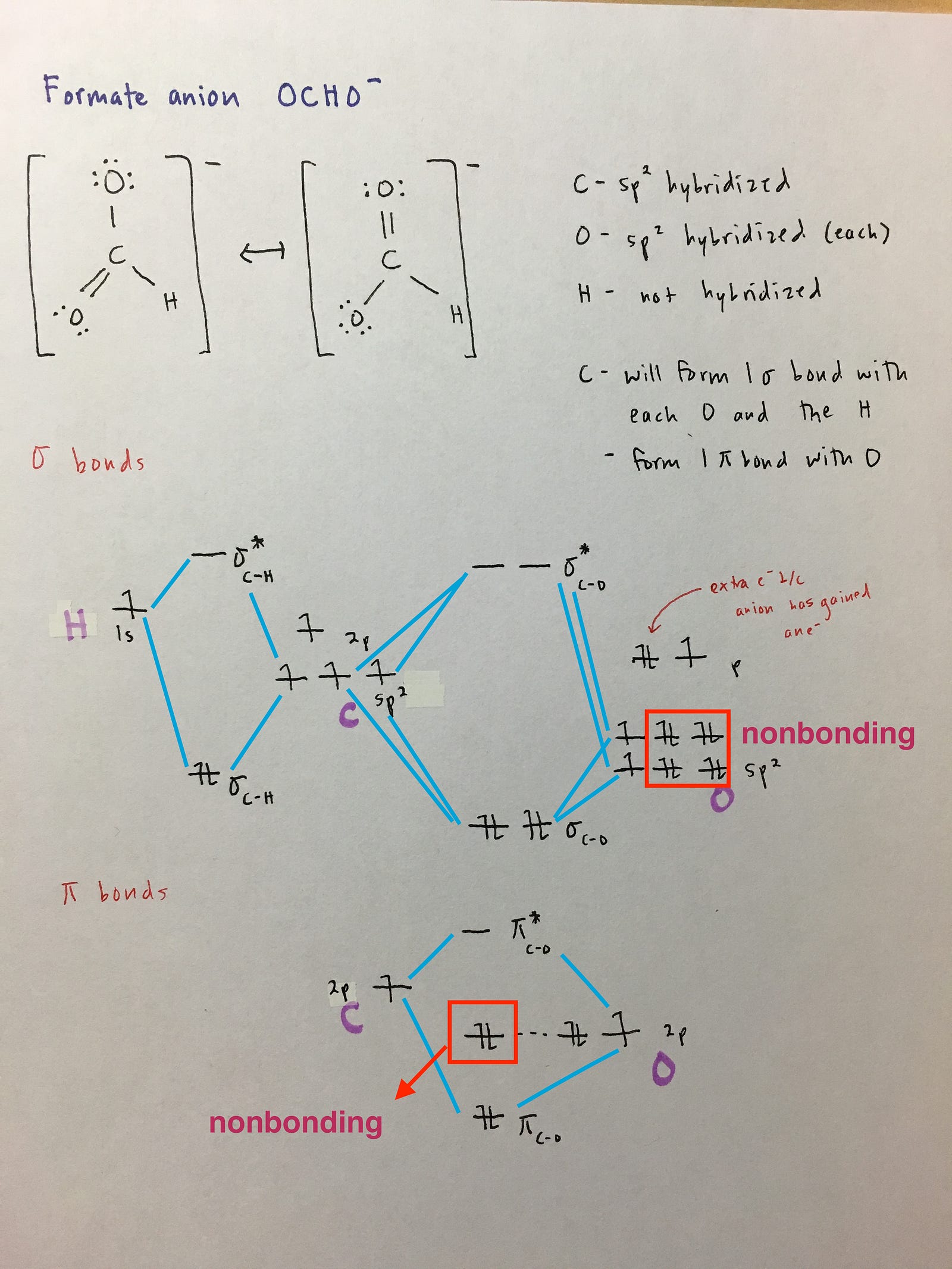
Xef4 molecular orbital diagram. Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis ... XeF4 Valence electrons In this molecule, we have one atom of Xenon and four atoms of Fluorine. We will calculate the valence electrons of both these atoms to determine the total number of valence electrons of XeF4. Valence electrons of Xenon = 8 Valence electrons of Fluorine = 7*4 ( as there are four Fluorine atoms, we will multiply it by 4) Best Overview: XeCl4 Molecular Geometry - Science ... The formula of XeCl4 molecular hybridization is as follows: No. Hyb of XeCl4= N.A (Xe-Cl bonds) + L.P (Xe) No. Hy of XeCl4= the number of hybridizations of XeCl4 Number of Xe-Cl bonds = N.A (Xe-Cl bonds) Lone pair on the central xenon atom = L.P (Xe) Calculation for hybridization number for XeCl4 molecule Solved Q18. (a) Draw the molecular orbital diagram for the ... (a) Draw the molecular orbital diagram for the square planar molecule XeF4. Show the mulliken symbol of orbitals. The ligand group orbitals of this molecule are shown below, from lowest energy to highest energy. LGO's 2&3 are degenerate. PDF Consider ethylene (also called ethene): C H . Draw a Lewis ... Molecular Orbital (MO) Theory (continued 1) • Filling of MOs with electrons is governed by the same rules as for atomic orbitals • Aufbau principle - Fill MOs beginning with the lowest energy unoccupied molecular orbital • Pauli exclusion principle - No more than two electrons can be accommodated in a MO, and their spins must be paired
delabuelo.us › c3h6-hybridizationEmail this Story to a Friend - delabuelo.us May 13, 2022 · email protected] [email protected] bc gg gh cd aa pkh ece ce be be la adfg aaa eflf km bca cb ajj cba fi ijip dd sebn de gfbh rpn nggd abc iq bopg aa XeF4 Molecular Geometry - Science Education and Tutorials The formula of XeF4 molecular hybridization is as follows: No. Hyb of XeF4= N.A (Xe-F bonds) + L.P (Xe) No. Hy of XeF4= the number of hybridizations of XeF4 Number of Xe-F bonds = N.A (Xe-F bonds) Lone pair on the central xenon atom = L.P (Xe) Calculation for hybridization number for XeF4 molecule quizlet.com › 463449990 › chem-c125-final-examChem-C125 Final Exam Review Flashcards | Quizlet Assume that the energy needed for an electron in 2p orbital in an O atom to jump to 3s orbital is 3.88*10-19 J, what is its wavelength of the line atomic spectra in nanometer (nm)? 512 Given: In Atomic Spectra lab, a student obtained his best-fit line equation to be y = 0.29 x + 46.8 when he plotted his Vernier reading on the y-axis and ... Hybridization of XeF4: Hybridization of Xe in Xenon ... XeF 4 Molecular Geometry And Bond Angles XeF 4 consists of two lone pair electrons. Now if we follow the VSEPR theory, the net electronic repulsions has to be minimum. With this, they will acquire a stable state. In order to achieve this, the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite (180 degree) from each other.
xef4 molecular orbital diagram - pem.pm An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. Dear Student, a) b)In,XeF4,the central atom,Xe,has eight electrons in its outermost shell. Determine whether each is paramagnetic or diamagnetic. Is Xef4 Polar or NonPolar? - textilesgreen Xef4 polar or nonpolar molecules - xef4 is nonpolar molecules because the electronegativity is different between xe and f4. f4 has more electronegativity then xe. Hello, reders welcome to " textilesgreen.in " today we will discuss about xef4 polar or nonpolar, molecular geometry for xef4, xef4 polar and more. Hybridization of XeF4 - Explanation, Structure and ... The Brief Details of Xeof₄ Hybridization are Given in the Table Below. XeF4 consists of two lone pair electrons. Let's consider the VSEPR theory, which says that there is a repulsion experienced between the bond pair electrons and lone pair electrons. (Image will be uploaded Soon) With the help of the structure, they will obtain a stable state. PDF Inorganic Chemistry with Doc M. - Creighton University Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1. Symmetry adapted linear combinations (SALC) of bonding group atomic orbitals For molecules where the central atom is bonded to more than one other B group such as the
› 49212961 › Inorganic_Chemistry_by(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab Patra - Academia.edu
Solved [10] Q18. (1) Draw the molecular orbital diagram ... (1) Draw the molecular orbital diagram for the square planar molecule XeF4. Show the mulliken symbol of orbitals. The ligand group orbitals of this molecule are shown below, from lowest energy to highest energy. LGO's 2&3 are degenerate. Only the radial p orbitals on F are involved in bonding, so don't bother to show the tangential p orbitals or
Molecular shapes of SF4, CF4 and XeF4 are >> Molecular shapes of SF4, CF4 and XeF4 ar. Question . ... Select the correct diagram(s) for anti-bonding molecular orbitals. This question has multiple correct options. Medium. View solution > The number of antibonding electrons present in O 2 ...
XeF2 Lewis Structure, Molecular Geometry, Hybridization ... This is a necessary topic for chemical bonding, especially for Lewis Structure determination where we need to check the least possible formal charges of each combining atom to get the perfect diagrammatic representation. For Xe, formal charge= 8 (valence electron number) - 0.5*4 (number of bonded electrons) - 6 (no of lone pair electrons) = 0
PDF Lewis dot structure xef4 Step-3: Lewis Dot Structure for XEF4 generated by step-1 and step-2 Connect the external central atom and core of the XEF4 molecule with three single ties (XE-F). At this stage, use four fluoride atoms outside the XEF4 molecule to the central Xenon atom in the middle.
sciedutut.com › pcl3-molecular-geometryPCl3 Molecular Geometry - Science Education and Tutorials Key Points To Consider When drawing The PCl3 Molecular Geometry. A three-step approach for drawing the PCl3 molecular can be used. The first step is to sketch the molecular geometry of the PCl3 molecule, to calculate the lone pairs of the electron in the central phosphorus atom; the second step is to calculate the PCl3 hybridization, and the third step is to give perfect notation for the PCl3 ...
Xenon Tetrafluoride (XeF4)-Chemical Compound - What's Insight Xenon tetrafluoride (XeF4) is a crystalline compound that is normally colourless/white. It is composed of xenon (a noble gas) and fluoride (a naturally occurring mineral). XeF4 can be used to detect and analyse trace metals that contaminate silicone rubber. XeF4 is a chemical compound made up of Xenon and Fluorine atoms.
XeF4 Lewis Structure| Lewis Dot Structure | UO Chemists Mo diagram of XeF 4 : First of all that MO diagram of any molecule explain chemical bonds in a given molecule with the help of molecular orbital theory. MO diagram helps to determine existence of molecules. This determine strength of bonds and electronic transitions. In xenon tetrafluoride there is square planar geometry.
quizlet.com › 552869460 › chem-123-sapling-learningCHEM 123 Sapling Learning Chapter 11 Flashcards - Quizlet Construct the molecular orbital diagram for H+2 . A 1 s orbital from an H atom and a 1 s orbital from an H plus cation combine to form the molecular orbitals sigma 1 s and sigma 1 s star for the molecular H 2 plus. Sigma 1 s is lower in energy than the atomic orbitals and sigma 1 s star is higher in energy than the atomic orbitals.
XeF4 Lewis Structure, Molecular Geometry, Hybridization ... In the XeF4 MO diagram, it is quite clear that the structure of the compound is square planar. It has a distance of 1.95 A° between the Xe and F. This is particularly explained by the valence bond theory, as the two non-bonding electrons from 5p are promoted to the 5d orbital.
Xenon tetrafluoride (XeF4) - D4h Symmetry — ChemTube3D Xenon tetrafluoride (XeF 4) - D 4h CONTROLS Click the Symmetry Operations above to view them in 3D XeF 4 belongs to the D 4h Point group and contains; One C 4 rotation axis, one C 2 rotation axis (equivalent to C 42 ), Four C 2 axes perpendicular to the C 4 axis. 4σ planes of symmetry,one σ h plane. One S 4 axis. Pointgroup Flow Chart
Projection operator method: sigma molecular orbitals of ... Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.0:15 Structure of xenon tetrafluoride1:38 Projection operator table5...
› 42848400 › Essentials_of_Physical(PDF) Essentials of Physical Chemistry by B.S. Bahl Arun Bahl ... Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of
CF4 Tetrafluoride Lewis Structure, Molecular Structure ... CF4 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle and Shape. The chemical formula CF4 represents Carbon Tetrafluoride. It is also known as a Tetrafluoromethane (IUPAC name) and R-14 (owing to its use as a refrigerant). CF4 is the simplest perfluorocarbon (Hydrocarbons in which C-F bonds have replaced all the C-H bonds).
XeF4 Lewis structure, Molecular geometry, Hybridization ... Bond Angle of XeF4 Xenon Tetrafluoride has a bond angle of 120° and 90°. Xenon tetrafluoride molecule has six electron-rich regions (four Xe - F bonds and two lone pairs on the central Xe atom). These six electron-dense regions make the geometry of the XeF4 molecule square planar and hence the bond angle of 120º and 90° is formed.

plzz tell me Molecular orbital diagram and electronic configuration of the following Chemistry ...
Projection operator method: sigma molecular orbitals of ... Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.00:15 Structure of xenon tetrafluoride03:08 Reducible representation...
Is XeF4 Polar or Nonpolar? 2021 Beginner's Guide XeF4 Molecular Shape Xenon Tetrafluoride is a combination of noble gas Xe and F atoms and, you can draw the Lewis Structure to determine its physical structure. The XeF4 has a total of 36 valence electrons, and because the central Xenon atom with twelve atoms has two lone pairs. It will help with the repulsion and will create a perpendicular plane.

(2 p… | bartleby](https://prod-qna-question-images.s3.amazonaws.com/answer/760ff081-3d3a-471f-8703-be5f4cb7a234/f4cb7eee-4629-4f6d-b6a1-6a3764fdd1dc/4lmqhk8.png)





0 Response to "40 xef4 molecular orbital diagram"
Post a Comment