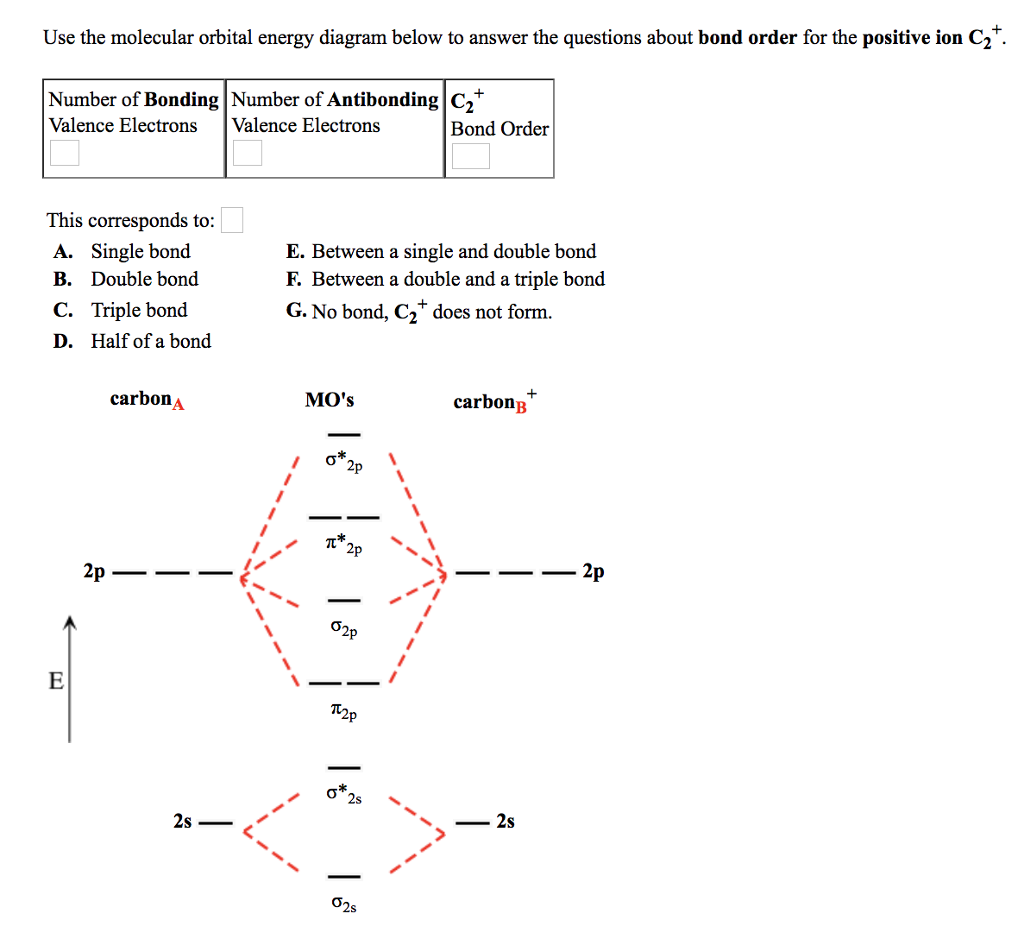
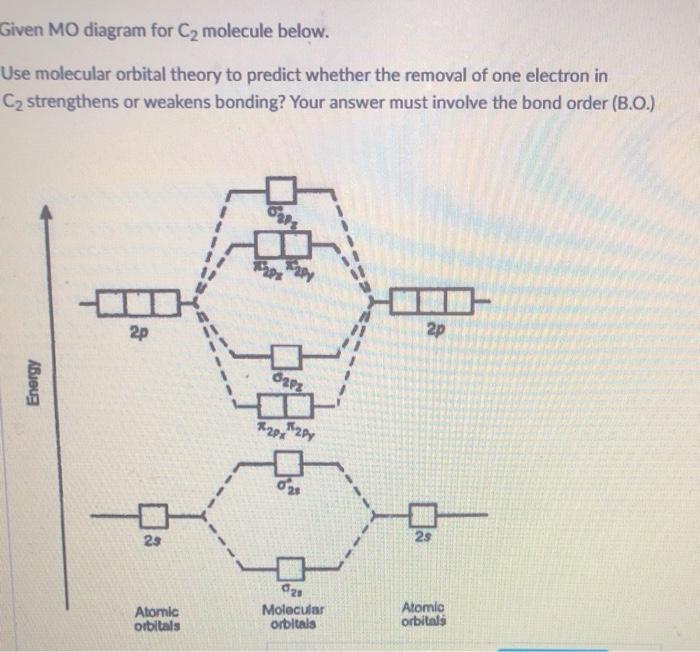
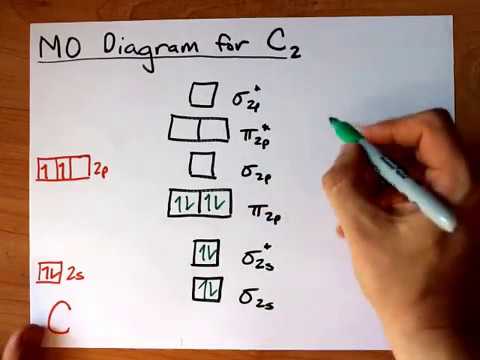
45 use the mo diagram provided below to answer the following questions: what is the bond order for c2?
TR_redirect – Defense Technical Information Center - DTIC To fix an outdated citation hyperlink: Take the alphanumeric code at end of the broken hyperlink and add to the end of the link. To find a specific citation by accession number: Take the accession number and add to the end of the link below. PDF Practice Test Questions 3 Molecular Orbital Theory: Heteronuclear ... energy level diagram for the helium protonate ion (𝐻𝐻𝐻𝐻𝐻𝐻+). Label all orbitals on your diagram, and include electrons in the AOs and MOs. You MUST draw sketches of the MOs as well as the energy levels. Include the effects of . polarization. (b) According to your MO diagram, what is the bond order for this ion?
Solved Use the MO diagram provided below to answer the - Chegg Question: Use the MO diagram provided below to answer the following questions: • What is the bond order for C2?_[Select] • Is C2 paramagnetic or diamagnetic ...
Use the mo diagram provided below to answer the following questions: what is the bond order for c2?
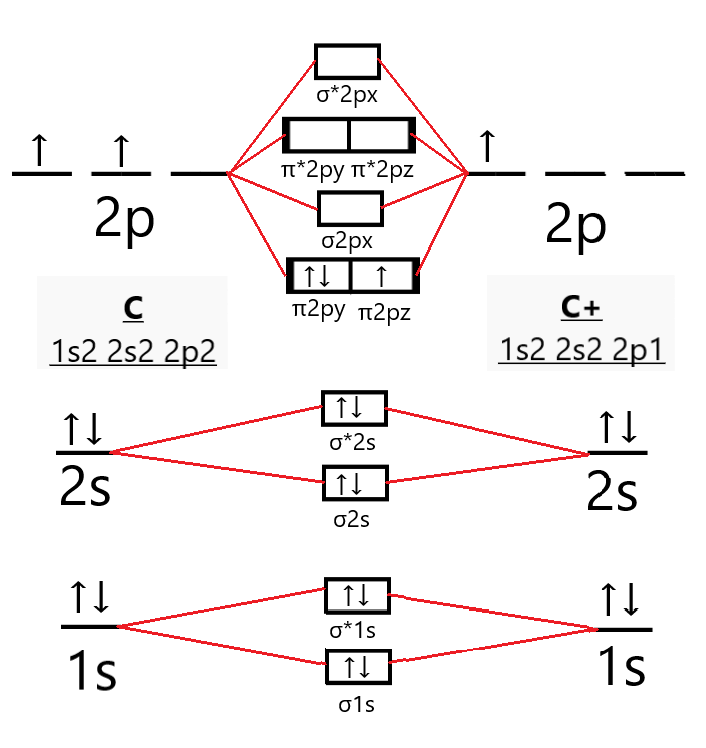
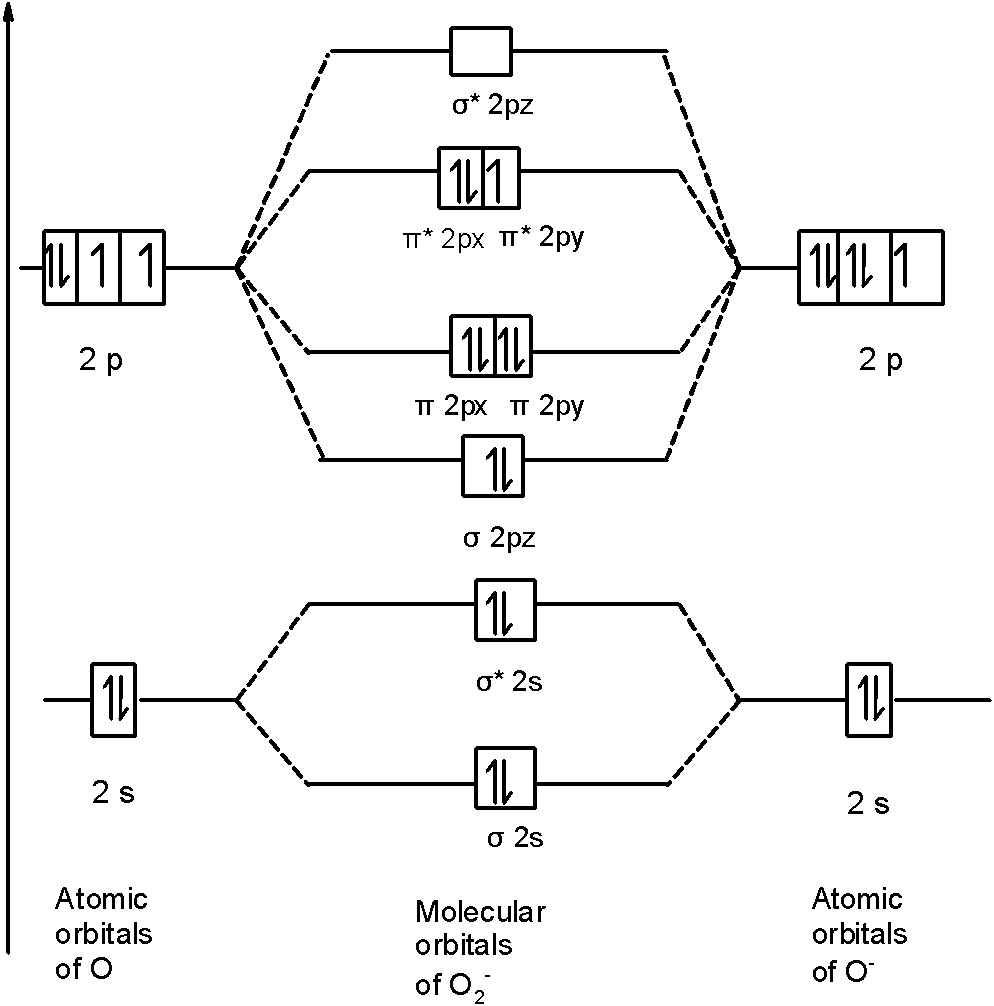
What is the molecular orbital diagram for O2- and O2+ ions? The following is the diagram for the neutral oxygen. The bond order is shown for the neutral oxygen. To figure it out for the positive ion simply remove one electron from the highest level, an antibonding orbital. So the positive ion has a higher bond order by 1/2 than neutral . For the negative ion. Bond order/strength/lengths, molecular geometry Flashcards | Quizlet What is the bond order of CO? Highest bond energy CO O2 F-OF Lowest bond energy Rank the following series of molecules or ions in order of decreasing bond energy using their bond order to predict relative magnitude: F-OF, O2, CO Longest bond F-OF O2 CO Shortest bond The bond order of C2+ is A 1 B 2 C dfrac32 D dfrac class ... - Vedantu From the molecular orbital diagram of the $C_{2}^{+}$ molecule we can see that it has 7 electrons in its bonding orbitals (${{N}_{b}}$) and 4 electrons in its ...
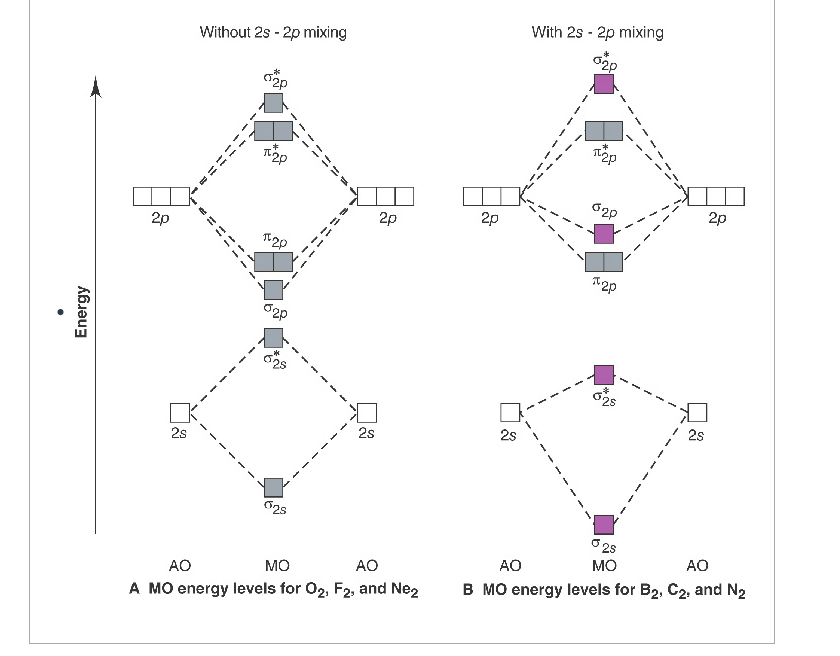
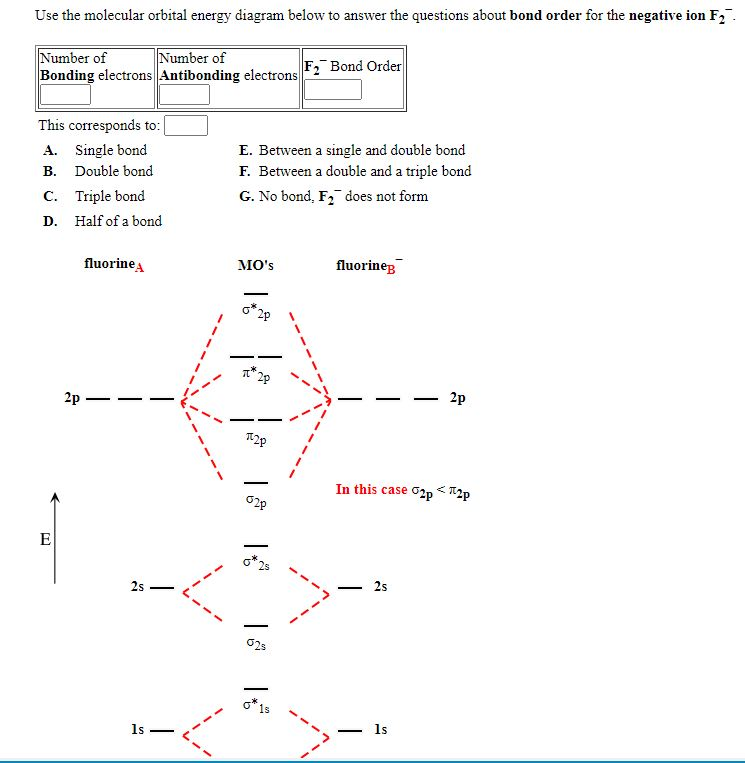
Use the mo diagram provided below to answer the following questions: what is the bond order for c2?. Molecular Orbital (MO) Diagram of C2 - YouTube Jun 9, 2017 ... Molecular Orbital Diagram for Carbon Dimer (C2).Fill from the bottom up, with 8 electrons total.Bonding Order is 2, and it is ... PDF Discussion Sheet 11 KEY - sdsuchem200.sdsu.edu Use the MO diagrams provided. 17. Refer to your MO diagrams. According to molecular orbital theory, what is the bond order for each of the following: a. C22- d. Li2 18. Refer to the MO Diagrams. According to molecular orbital theory, which of the following lists ranks the fluorine species in terms of increasing bond order? d. F2 < F22+ < F22— a. Answered: Answer the following questions and use… | bartleby Q: 1) Construct an MO diagram for the formation of Ne, (atomic No. of Ne = 10) use only the valence… Q: 1. The hybridization of a chiral center must b [Select ] sp sp2 sp3 Q: Which statement is true according to molecular orbital (MO) theory. 2020 (a) Mixing an odd number of… A: The key features of the molecular orbital theory are listed below. PDF MO Diagrams for Diatomic Molecules - University of California, Irvine Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Give the Molecular Orbital Energy diagram of a) N2 - - and b)O2 Click here to get an answer to your question ✍️ Give the Molecular Orbital Energy diagram of a) N2 and b) O2 . Calculate the respective bond order. [Solved] Using the correct MO diagram (pick option A or B from the ... Answer given below Step-by-step explanation * please give helpful kindly Image transcriptions For C, the MO diagram is option B that is PP AV 6 y Qs bond ofder = 6- 2 2 - =2 F the MO diagram is option A , that is 5u AV Ps 6 u AV 8 - 6 Bond order = 2 =1 As ( 2 has bond order 2 so it has the shorter bond length Hence answer Is Cy 2 Attachments jpg write molecular orbital configuration of c2 predict ... - TopperLearning The molecular orbital diagram for C 2 molecule is : The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. Bond order ofO2 O2+ O2 and O22 is in order A O2 langle class ... - Vedantu Therefore, the bond order. O 2 2-. is 1. So, the correct order of bond order is O 2 2- O 2 − O 2 O 2 +. So, the correct answer is "Option B". Note: You should notice that bond order is indirectly proportional to the length of the bond. The higher the bond order, the shorter and stronger will be the bond.
BibMe: Free Bibliography & Citation Maker - MLA, APA, Chicago ... BibMe Free Bibliography & Citation Maker - MLA, APA, Chicago, Harvard IS LM Model Questions and Answers | Homework.Study.com In the IS-LM model, a policy is said to be ineffective in the long run because it cannot change A. the price level. B. output. C. the interest rate. D. all of the above. View Answer. The LM curve will shift to the left if the output is {Blank} its natural rate. A. above B. below C. equal to D. cannot be determined. What is the bond order for C2? Select ] Is Cz paramagnetic or ... Dec 9, 2021 ... Sara F. Chemistry 101. 5 months, 3 weeks ago. Use the MO diagram provided below to answer the following questions: What is the bond ... Use the MO diagram provided below to answer the following questions ... Use the molecular orbital energy diagram below to answer the questions about bond order for the positive ion C2 Number of Bonding Number of Antibonding C2 Valence Electrons Valence Electrons Bond Order This corresponds to A. Single bond B. Double bond C. Triple bond D. Half of a bond E. Between a single and double bond F.
(PDF) MODERN PRINCIPLES OF ECONOMICS | J Prins - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
Purple Mash by 2Simple Purple Mash is an award-winning website for nursery and primary school children. It enables children to explore and enhance their knowledge in a fun and creative way.
TR_redirect – Defense Technical Information Center - DTIC Although the DTIC may or may not use these sites as additional distribution channels for Department of Defense information, it does not exercise editorial control over all of the information that you may find at these locations. Such hyperlinks are provided consistent with the stated purpose of this website. The DTIC bears no responsibility for ...
What is the bond order of a C2 molecule? - Quora According to molecular orbital theory it is 2. Your response is ...
CHM Final Flashcards | Quizlet Use the diagram below to answer the following questions. From the phase diagram above, the minimum pressure at which this substance can exist in the liquid phase is 0.45 atm
Objective Proficiency. Student's Book 2ed, 2013 280p Enter the email address you signed up with and we'll email you a reset link.
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules - CPP Bond order is a simple calculation, based on the number of bonding versus antibonding electrons ... There would be four electrons to fill into our molecular orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO. What we gain in the bonding sigma MO, we lose in the anti-bonding sigma-star MO ...
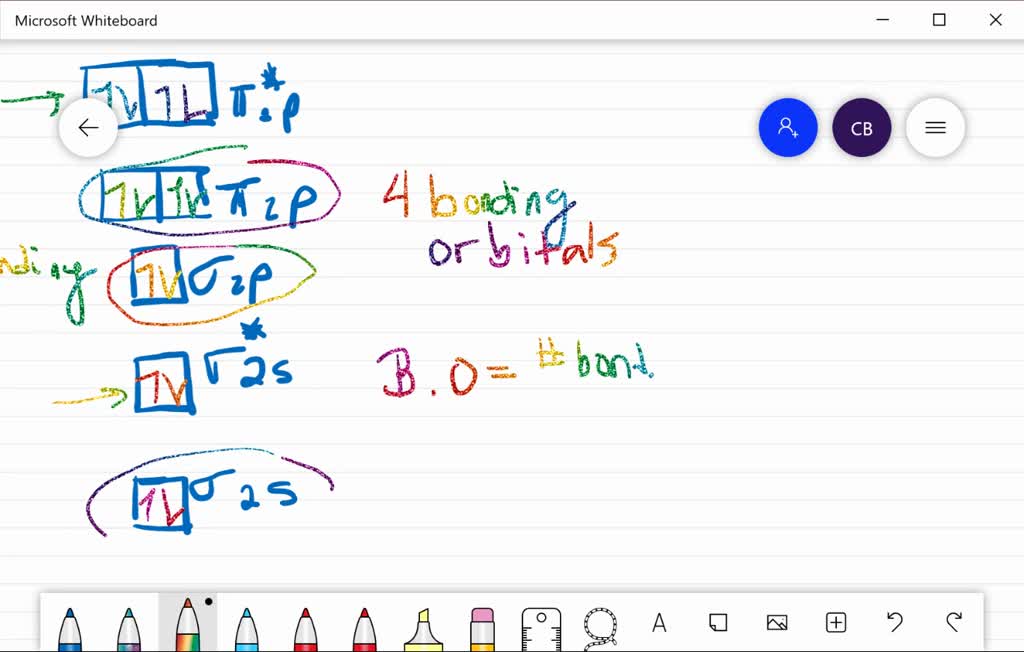
use the mo diagram provided below to answer the following questions what is the bond order for c2 select is cz paramagnetic or diamagnetic select what is the bond order for c2 select is cz p 66533
Problem 32E from Chapter 11 - Chegg The ion has valence These valence are assigned to the molecular orbital's shown as: Bond order . has more number of bonding and bond order is has one unpaired Hence it is paramagnetic . Chapter 11, Problem 32E is solved. View this answer View this answer View this answer done loading. View a sample solution. Step 2 of 3. Step 3 of 3. Back to top.
Answered: Construct the molecular orbital diagram… | bartleby Identify the bond order. Answer Bank 11 2p 2p 2p 0.5 1 1.5 2.5 2.s. Skip to main content. close. Start your trial now! First week only $4.99! arrow_forward. Literature guides Concept explainers Writing ... Construct the molecular orbital diagram for N,. Identify the bond order. Answer Bank 11 2p 2p 2p 0.5 1 1.5 2.5 2.s
Answered: To answer the next several questions,… | bartleby To answer the next several questions, sketch the MO diagrams for the following molecules: 02. 022+, 022, 02. Which of the following statements is true? Only o22- is paramagnetic. 02 and 02 are both paramagnetic. Only O2 is paramagnetic. Only 022* is paramagnetic. Question
Molecular Orbital (MO) Diagram for C2(2-) - YouTube Aug 3, 2020 ... When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond ...
Part A Use the drawing of the MO energy diagram to predict the bond ... Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
Post Lecture Lewis Structures, Problem Set #6 (Ch 5), Problem Set #7 ... Terms in this set (24) Draw the Lewis structure for CO. The most stable structure for CO (carbon monoxide) allows for the octet of each atom to be complete without introducing a formal charge. Based on the valence numbers of the atoms, the Lewis structure will contain 10 electrons. Note that, for carbon monoxide to follow the octet rule, both ...
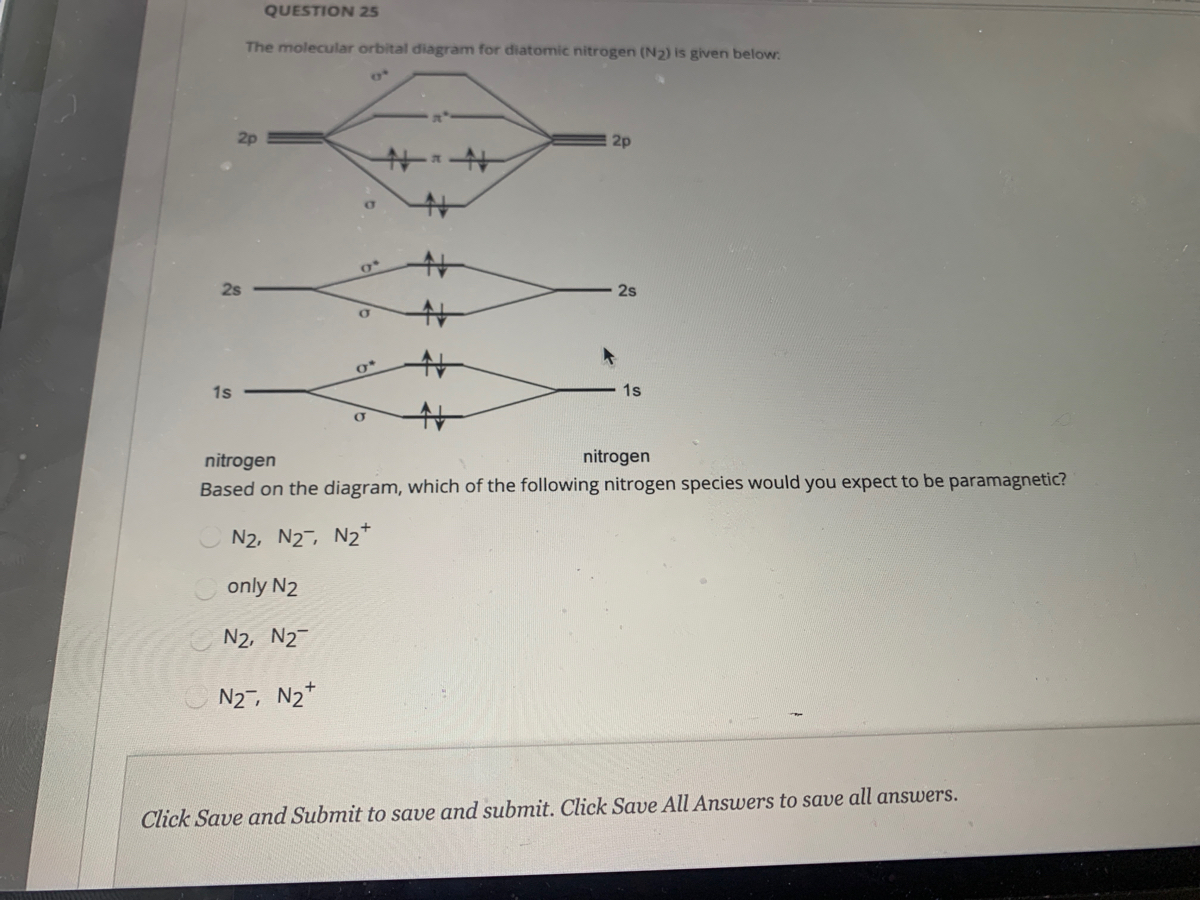
Solved Use the MO diagram provided below to answer the - Chegg Expert Answer. 94% (18 ratings) Transcribed image text: Use the MO diagram provided below to answer the following questions: • What is the bond order for N? [Select] • Is N2 paramagnetic or diamagnetic?__ (Select] netic? _ [ Select] • What is the bond order for N?
Answered: Question 2 of 25 Submit Use the MO… | bartleby Q: For the following molecules or ions, 1) calculate the bond order.2) state whether the molecule or… A: Since you have posted questions with multiple sub-parts, we are entitled to answer the first 3 only.…
CHEM 123 Sapling Learning Chapter 11 Flashcards | Quizlet Each carbon atom is bonded to three other atoms in anthracene. When bonded to three other atoms, carbon is 𝑠𝑝2 hybridized. Single bonds are σ bonds. Double bonds are made up of one σ bond and one π bond. There are 19 single bonds and 7 double bonds in this molecule, so there are 19+7=26 σ bonds.
Use the molecular orbital energy level diagram to show that N2 - Toppr Click here to get an answer to your question ✍️ Use the molecular orbital energy ... Bond order indicates the number of bonds in diatomic molecule is 3, ...
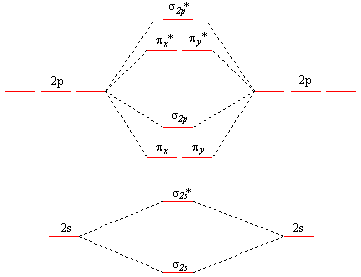
MO Diagrams - GitHub Pages In a diatomic molecule such as O2 O 2, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: Bond Order = 1 2 [(Bonding e−) − (Antibonding e−)] Bond Order = 1 2 [ ( Bonding e -) - ( Antibonding e -)]
PDF 100 Practice Questions for Chem 1C Midterm 1 - Joseph Use the following to answer question 9: Draw the Lewis structures of the molecules below, and use them to answer the following questions. I. BH 3 II. NO 2 III. SF 6 IV. O 3 V. PCl 5 9. Which of these molecules show resonance? A) I, II B) II, IV C) II, V D) III, IV E) III, V 10. Which of the following has an incomplete octet in its Lewis structure?
What is the molecular orbital diagram for C_2^-? | Socratic Explanation: The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add an electron to that diagram. You need to add an electron and not remove one because of the overall negative charge that exists on the molecule. As you know, a neutral carbon atom has a total of 6 electrons.
Answered: Jse the MO diagram (below) to calculate… | bartleby Solution for Jse the MO diagram (below) to calculate the bond order for CO. - Op Op 1 3 4 C 7 8 9. +/- х 100 2. Skip to main content. close. Start your trial now! First week only $4.99! arrow_forward ... Science Chemistry Q&A Library Jse the MO diagram (below) to calculate the bond order for CO. - Op Op 1 3 4 C 7 8 9. +/- х 100 2.
(PDF) MODERN PRINCIPLES OF ECONOMICS | J Prins Enter the email address you signed up with and we'll email you a reset link.
HW Ch 11 Flashcards | Quizlet 0.5. Which statement for NH3 and NF3 is false? Electronegativities: N = 3.0, H = 2.1, F = 4.0. The bond angles in NF3 are smaller than those in NH3. Both are sp3 hybridized at nitrogen. The bond dipoles of NF3 are directed toward fluorine, whereas those in NH3 are directed toward nitrogen. The bond dipoles in NF3 are directed toward the more ...
The bond order of C2+ is A 1 B 2 C dfrac32 D dfrac class ... - Vedantu From the molecular orbital diagram of the $C_{2}^{+}$ molecule we can see that it has 7 electrons in its bonding orbitals (${{N}_{b}}$) and 4 electrons in its ...
Bond order/strength/lengths, molecular geometry Flashcards | Quizlet What is the bond order of CO? Highest bond energy CO O2 F-OF Lowest bond energy Rank the following series of molecules or ions in order of decreasing bond energy using their bond order to predict relative magnitude: F-OF, O2, CO Longest bond F-OF O2 CO Shortest bond
What is the molecular orbital diagram for O2- and O2+ ions? The following is the diagram for the neutral oxygen. The bond order is shown for the neutral oxygen. To figure it out for the positive ion simply remove one electron from the highest level, an antibonding orbital. So the positive ion has a higher bond order by 1/2 than neutral . For the negative ion.











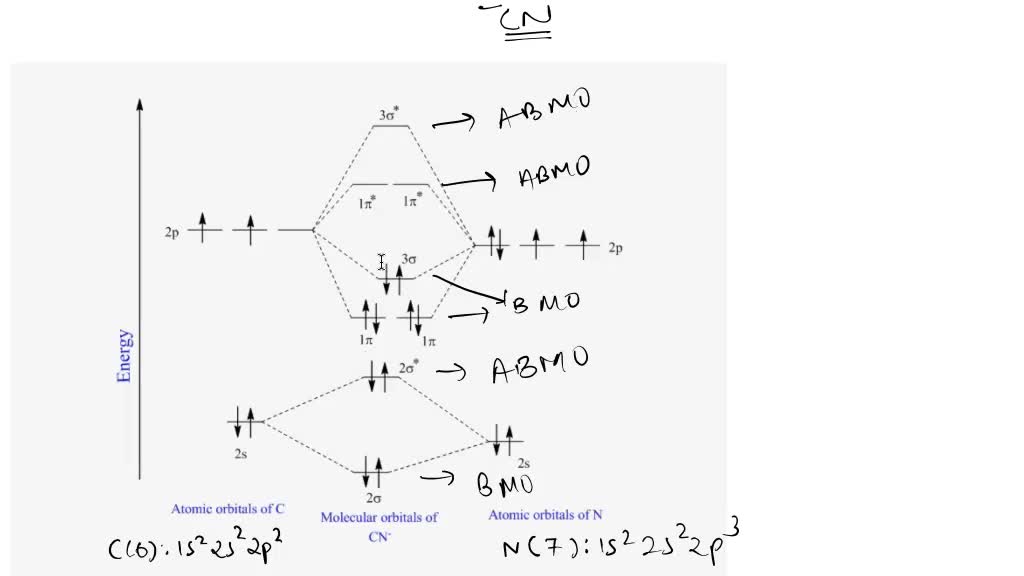
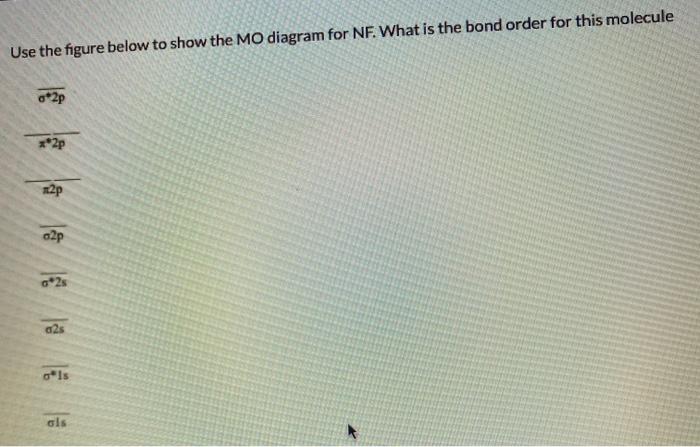
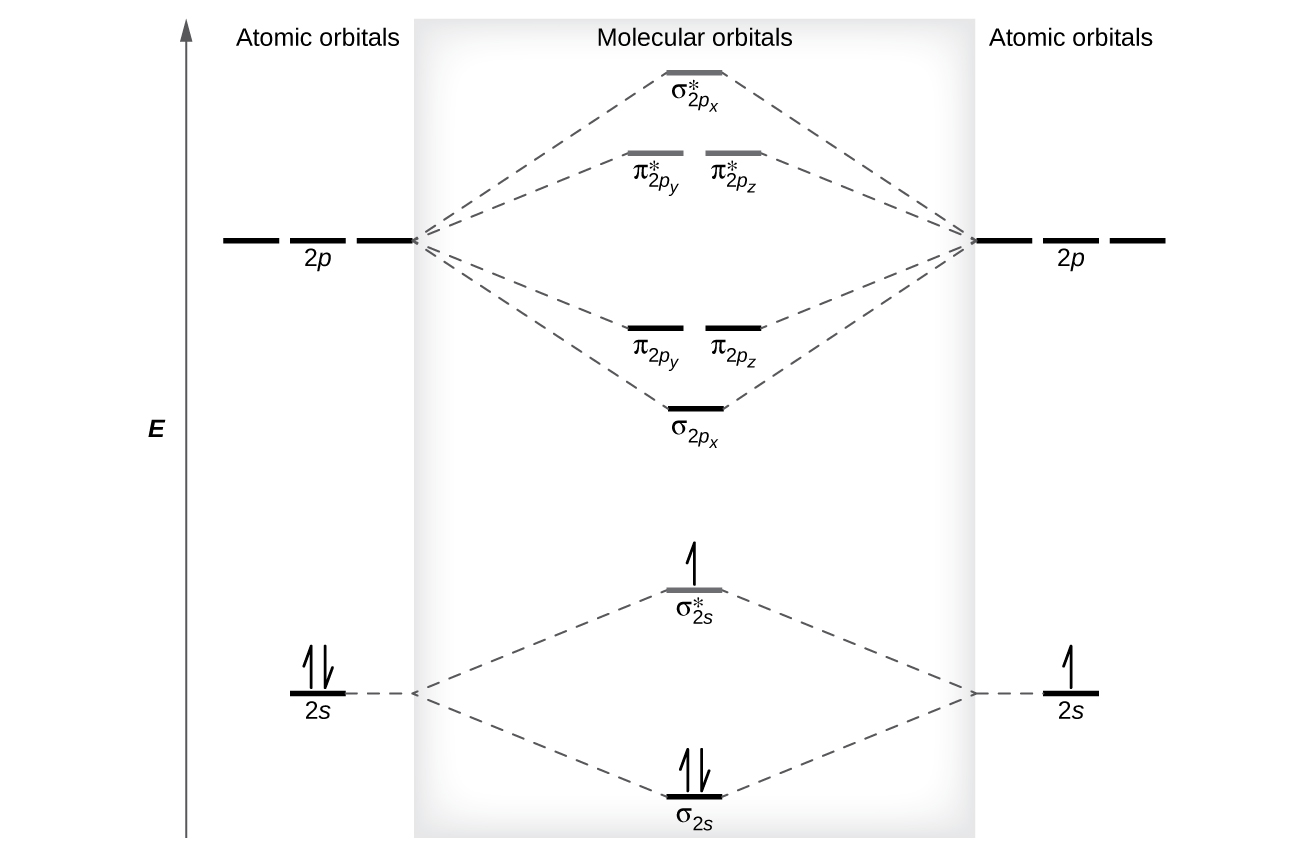

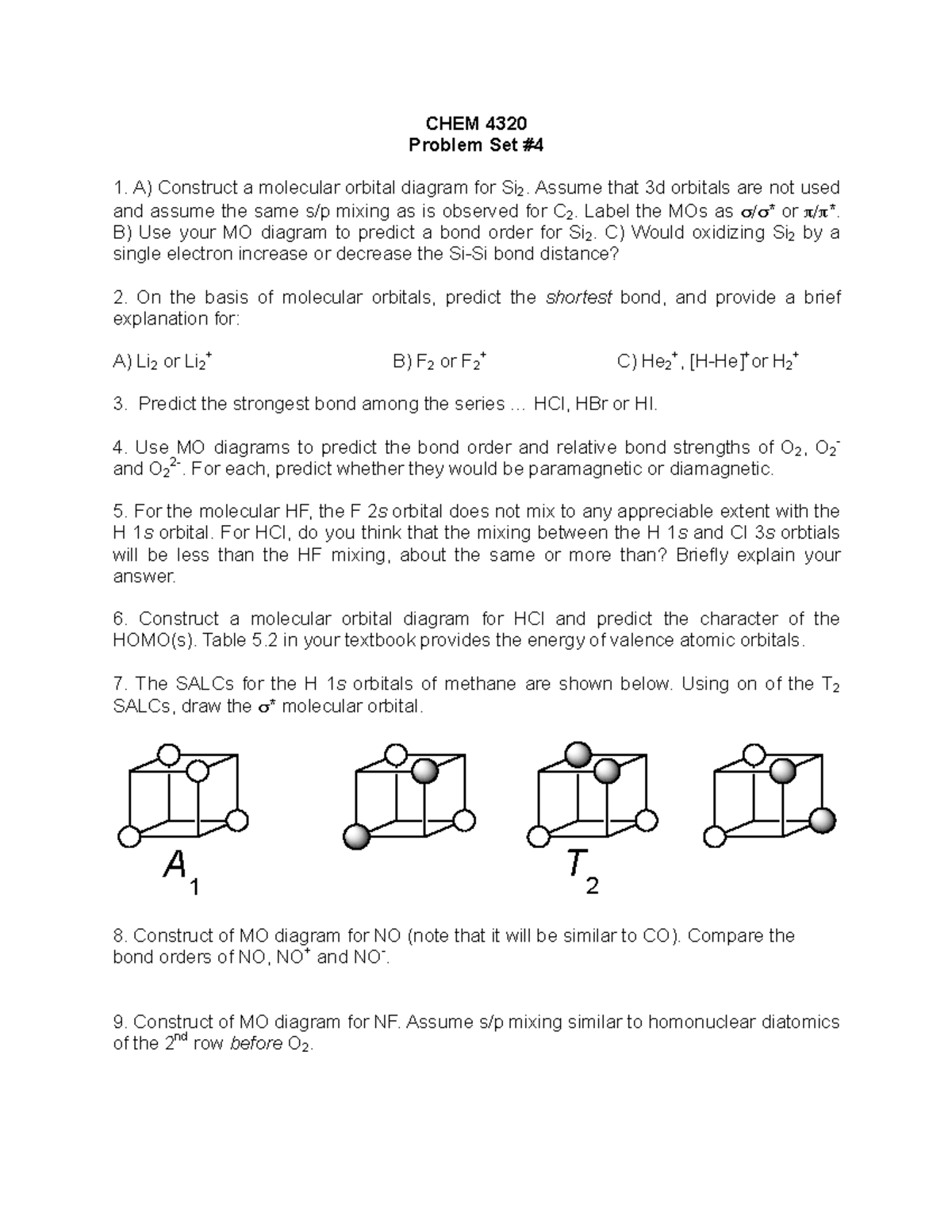

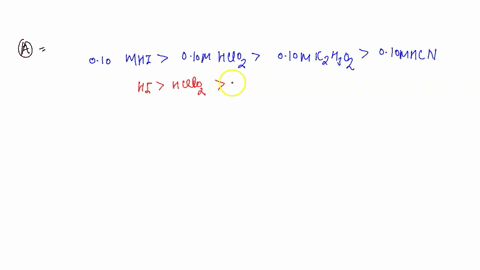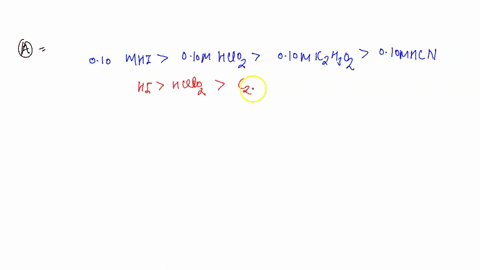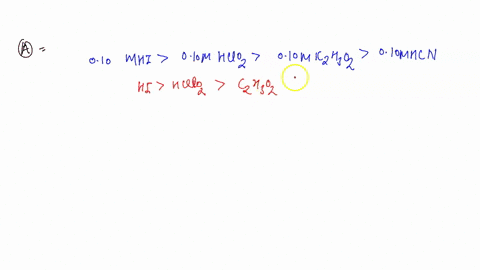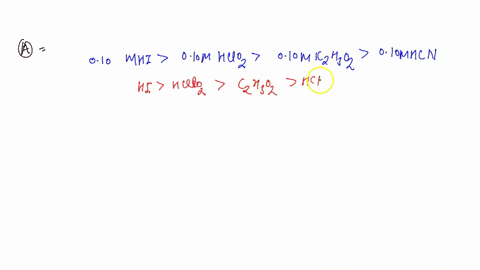


0 Response to "45 use the mo diagram provided below to answer the following questions: what is the bond order for c2?"
Post a Comment