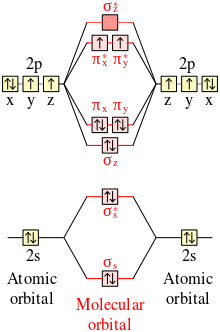
44 cyanide molecular orbital diagram
Q1-) Draw the molecular orbital energy diagram of the… - SolvedLib a. Prepare a molecular orbital energy-level diagram for the cyanide ion. Use sketches to show clearly how the atomic orbitals interact to form MOs. b. What is the bond order for cyanide, and how many unpaired electrons does cyanide have? c. CN Lewis Structure, Molecular Geometry, Hybridization ... 1 day ago · Molecular Orbital Diagram. Molecular Orbital Theory is slightly different from VBT and orbital hybridization. Here, AOs from different atoms inside the molecule can come together to form molecular orbitals or MOs. Therefore, valence electrons are shared inside the molecule. The electronic configuration of both C and N are as follows: Carbon ...
Cyanide complexes in Molecular Orbital Theory 1 Answer. In this answer of Martin's, you can find a molecular orbital diagram of C O. The corresponding diagram for cyanide, C N X − is essentially identical, there will only be different orbital energies and very slightly different extends of the lobes. When forming a coordinate bond to a metal centre, cyanide will primarily attack with ...

Cyanide molecular orbital diagram
Molecular cloud - Wikipedia A vast assemblage of molecular gas that has more than 10 thousand times the mass of the Sun is called a giant molecular cloud (GMC). GMCs are around 15 to 600 light-years (5 to 200 parsecs) in diameter, with typical masses of 10 thousand to 10 million solar masses. [10] Molecule - Wikipedia Molecular science. The science of molecules is called molecular chemistry or molecular physics, depending on whether the focus is on chemistry or physics.Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties. Problem 58 1 a prepare a molecular orbital diagram - Course Hero Problem 5.8 1: (a) Prepare a molecular orbital diagram for the cyanide ion. Use sketches to show clearly how the atomic orbitals interact to form MO's. (b) What is the bond order, and how many unpaired electrons does cyanide have? (c) Which molecular orbital of CN - would you predict to interact most strongly with a hydrogen 1 s orbital to form an H-C bond in the reaction CN - + H + HCN?
Cyanide molecular orbital diagram. 4.3: High Spin and Low Spin Complexes - Chemistry LibreTexts Jan 28, 2022 · Then, the next electron leaves the 3d orbital and the configuration becomes: [Ar]4s 0 3d 5. Thus, we can see that there are five electrons that need to be apportioned to Crystal Field Diagrams. The pairing of these electrons depends on the ligand. Since Cyanide is a strong field ligand, it will be a low spin complex. Cyanide ion | CN- - PubChem Cyanide ion | CN- | CID 5975 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem ... Solved STEP 2 OF BUILDING A MOLECULAR ORBITAL DIAGRAM FOR | Chegg.com Transcribed image text: STEP 2 OF BUILDING A MOLECULAR ORBITAL DIAGRAM FOR THE CYANIDE ION (CN) To develop our MO diagram, we need to consider the valence atomic orbitals of the carbon and nitrogen atoms. Both the symmetry and energy of these atomic orbitals will be important. For all steps in building the molecular orbital diagram, assume that the C-N bond lies along the z-axis Rank the ... Solved 2. Prepare a molecular orbital diagram for cyanide, - Chegg Transcribed image text: 2. Prepare a molecular orbital diagram for cyanide, CN. Use sketches to show clearly how the AOs interact to form MOs. Use the MO diagram to answer the following questions: a) What is the bond order for cyanide, and how many unpaired electrons does cyanide have?
Cyanide Ion (CN-): Lewis Structure, Molecular Geometry, Hybridization ... Determine whether the molecule has hetero atomic orbital or homo atomic orbital. Surely, cyanide ion (CN -) has a hetero-atomic molecular orbital (as it contains two different atoms). Fill molecular orbital by taking into account its energy (i.e.; bonding molecular orbitals are kept low than anti-bonding orbitals). Now, draw the MO diagram ... Cyanide (CN-) lewis structure, Molecular orbital diagram ... Clearly, Cyanide (CN) lies in a hetero-nuclear diatomic molecular orbital as it contains two different atoms. Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. SOLVED: Below is a reaction from organic chemistry. Based on ... Oct 27, 2022 · Below is a reaction from organic chemistry. Based on your molecular orbital diagrams from questions 2 and 3 above relating to formaldehyde and cyanide, explain why these species react in the way they do. Use the terms highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). HCN(Hydrogen Cyanide); Lewis Structure, Molecular Geometry ... Surely, hydrogen cyanide has a hetero-atomic molecular orbital (as it contains different atoms). Fill molecular orbitals by taking into account their energy (i.e., bonding molecular orbitals are kept lower than anti-bonding orbitals). Now, draw the MO diagram for hydrogen cyanide and fill the molecular orbital with electrons.
Chem 250 Lewis Structures Flashcards | Quizlet What is the correct molecular geometry of NH₃ according to Valence Shell Electron Pair Repulsion Theory? Trigonal pyramidal (pyramidal). Ammonia has three groups and one set of lone pairs bound to a central atom, which affords a trigonal pyramidal, or pyramidal, molecular geometry based on Valence Shell Electron Pair Repulsion Theory. Molecular Orbital Theory Heteronuclear Diatomic (Cyanide, CN ... - YouTube Dr. Shields shows you how to draw the MO correlation diagram for cyanide (CN-), calculate the MO bond order, and write the MO electron configuration with an ... What do the molecular orbitals of cyanide look like, compared ... - Quora Answer: These two compounds have nearly the same atomic orbitals and very similar bond distances. In a nitrile group, the C-N bond length is 1.160 angstroms long, while the C-O bond in carbon monoxide is 1.114 angstroms. Both have very strong bonds, requiring tremendous amounts of energy to bre... SOLVED:Draw the molecular orbital diagram for the cyanide ... - Numerade Um, they Mm. Atomic orbital molecular diagram is to be launched three sigma one by one by creep by one by and one bye to be Then the second live made So sigma the West. Okay, go by two sigma and the land Thirties one Sigma bond did just be oneness Atomic Hi, Bedell Off nitrogen one by molecular orbital off, But not yeah, one atomic or we tell off.
SOLVED:a. Prepare a molecular orbital energy-level diagram for the ... Draw a molecular orbital diagram for cyanide CN, CN-, and CN+. Give the bond or… 06:08. a. Prepare a molecular orbital energy-level diagram for NO, showing clearly how… 02:58. Use molecular orbital theory to predict the arrangement of electrons in MOs, th… 03:44 (a) Sketch the molecular orbitals of the $\mathrm{H}_{2}^{-}$ ion and draw ...
The Molecular orbitals of the Cyanide anion - ch.ic.ac.uk The Molecular orbitals of the Cyanide anion. The display shows the five occupied valence molecular orbitals and three unoccupied orbitals. The Calculation was done at the following level: B3LYP/6-31G (d). A tutorial on how to construct the diagram from the starting atomic orbitals. Note in particular the odd form of the 6σ antibonding orbital.
STEP 2 OF BUILDING A MOLECULAR ORBITAL DIAGRAM FOR | Chegg.com Transcribed image text: STEP 2 OF BUILDING A MOLECULAR ORBITAL DIAGRAM FOR THE CYANIDE ION (CN) To develop our MO diagram, we need to consider the valence atomic orbitals of the carbon and nitrogen atoms. Both the symmetry and energy of these atomic orbitals will be important. For all steps in building the molecular orbital diagram, assume that the C-N bond lies along the z-axis Rank the ...
Problem 58 1 a prepare a molecular orbital diagram - Course Hero Problem 5.8 1: (a) Prepare a molecular orbital diagram for the cyanide ion. Use sketches to show clearly how the atomic orbitals interact to form MO's. (b) What is the bond order, and how many unpaired electrons does cyanide have? (c) Which molecular orbital of CN - would you predict to interact most strongly with a hydrogen 1 s orbital to form an H-C bond in the reaction CN - + H + HCN?
Molecule - Wikipedia Molecular science. The science of molecules is called molecular chemistry or molecular physics, depending on whether the focus is on chemistry or physics.Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties.
Molecular cloud - Wikipedia A vast assemblage of molecular gas that has more than 10 thousand times the mass of the Sun is called a giant molecular cloud (GMC). GMCs are around 15 to 600 light-years (5 to 200 parsecs) in diameter, with typical masses of 10 thousand to 10 million solar masses. [10]

0 Response to "44 cyanide molecular orbital diagram"
Post a Comment