41 molecular orbital diagram for bh3
Molecular orbital diagram for BF3 - Chemistry Stack Exchange This allows us to write the irreps of the three F 's orbitals. 3 F: a 1 ′ + a 2 ′ + a 2 ″ + 2 e ′ + e ″ With all the formalities out of the way, we can construct the qualitative MO diagram for B F X 3. 5. Construct a molecluar orbital diagram for BH3. Use | Chegg.com Construct a molecluar orbital diagram for BH3. Use these guidelines. i) Count the valence electrons in the molecule. ii) Check if any atoms need to be hybridized. iii) Construct the MO diagram by mixing atomic orbitals to form the same number of new molecular orbitals. The valence orbitals for B and H are energetically similar. iv) Draw an ...
Consider the borane-ammonia addition compound, | Chegg.com Consider the borane-ammonia addition compound, BH3NH3. Draw a molecular orbital energy level diagram for BH3NH3 with the BH3 orbitals on the left, the NH3 orbitals on the right, and the orbitals of BH3NH3 in the center. In which molecular orbital of BH3NH3 does the donor-acceptor interaction take place?

Molecular orbital diagram for bh3
5.5A: \(BH_3\) - Chemistry LibreTexts Hydrogen has higher electronegativity than boron, therefore hydrogen would have lower energy level in the MO diagram. -In addition, B has 3 electrons in the valence electrons and 3 hydrogens have total 3 electrons. Therefore, the total number of electrons filled in orbitals are 6. MO diagram of "B"_2"H"_6? + Example - Socratic.org For this we will get 1 + 1 + 1 + 1 + 1 +1 +1 +1 = 8. nˆR is the coefficient next to each symmetry operation (next to ˆE, ˆC2(z), ˆC2(y), etc). This is 1 in this case for all operations. Γbasis is the reducible representation. The "basis" will be either the 2s, 2px, 2py, or 2pz orbitals of boron, or the 1s orbitals of hydrogen. Lab 5 creating walsh diagrams from computed molecular orbital ... - StuDocu Lab 5 creating walsh diagrams from computed molecular orbital energies lab report by Hanna Thomson. Course:Inorganic Chemistry (CHEM 314) Hanna Thomson. Lab 5. W ed. 4pm. Lab 5: Cr eating W alsh Diagr ams from C omput ed Molecular Orbital. Ener gies . Background .
Molecular orbital diagram for bh3. MO of BH3.mpg - YouTube An advanced molecular orbital diagram of BH3 (borane) for the inorganic or physical chemistry student. MO Diagram of BH3 | Molecular orbital theory | Ligand groups of ... to join our neet/iit jee/iit-jam/csir-net/iit-gate/du/bhu/rpsc online regular/crash course please download the app and get registered there...app link-http:/... Why does BH3 exist as B2H6? - Quora Answer (1 of 2): * As we see Boron has 3 electrons in its valence shell. * The compound BH3 formation by vbt is shown in below figure, * Now we see that in valence shell ofBH3 there are 6 electrons. * But generally speaking about compounds, they are stable when they attain octate configuratio... (Get Answer) - Draw a molecular orbital diagram for borane, BH3 ... Draw a molecular orbital diagram for borane, BH3, assuming a trigonal planar geometry. Follow the model we did in class for pyramidal ammonia. Show all orbitals at their correct energy levels, including any and all bonding orbitals, antibonding orbitals, nonbonding orbitals, and atomic orbitals. Note that B is slightly less electronegative than ...
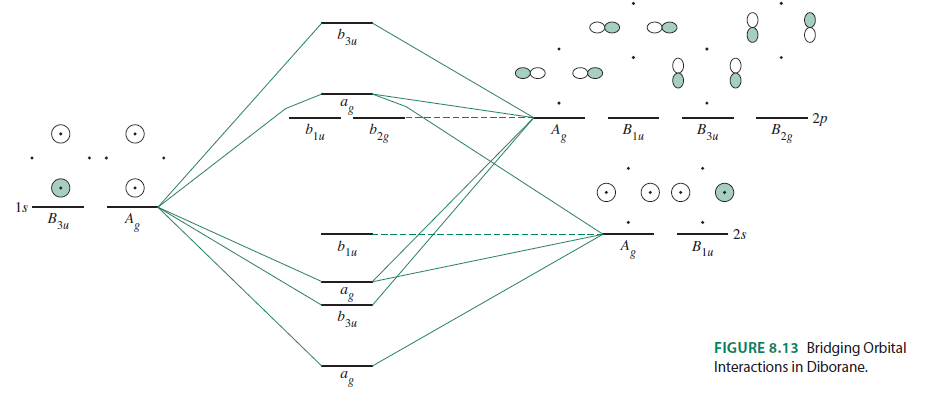
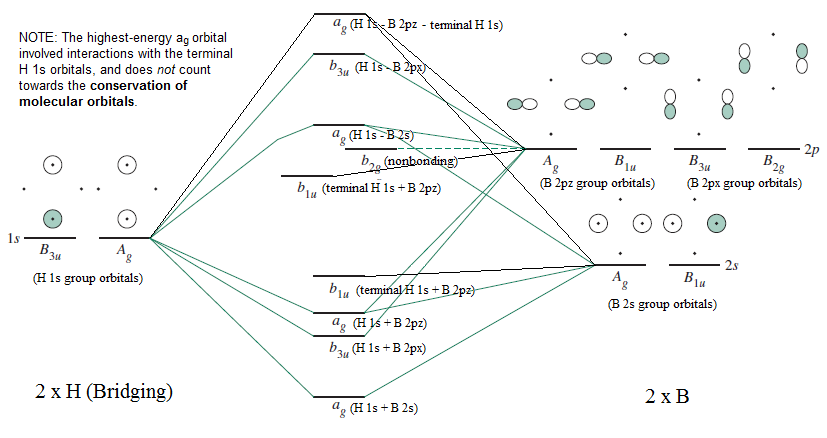
PDF Diborane - B2H6 - University of California, Los Angeles one sp3 orbital from each of the B atoms combines (Figure 3) with the 1s orbital of the bridging H atom to form three new molecular orbitals (MOs) - as always, n atomic orbitals (AO) form n MOs. One B atom gives its remaining valence electron to one bridge, and the other B atom gives to the other. Each bridge, Molecular orbital energy diagram for BH 3 - ResearchGate These molecules serve to illustrate the concept of ligand group orb... Context in source publication Context 1 ... a1' = 1, a e' = 1 Therefore, the symmetries of the LGOs for BH 3 are a 1 ' + e'.... Solved , construct the molecular orbital diagram for BH3. - Chegg Question: , construct the molecular orbital diagram for BH3. b)Based on your MO diagram explain why this molecule is a Lewis acid. c) Draw the MO diagram that we did in class for NH3. d) Draw the electron dot model for the product of the reaction between BH3 and NH3. e) Using your MO diagrams, which orbitals are involved in the latter interaction? Molecular Orbitals for BF3 - Newcastle University Molecular Orbitals for BF3. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital circle in the energy level correlation diagram shown The energy level diagram may be displayed with or without the group theory symbols and character table: the models accessed by clicking are the same.
BH3Orbitals - Yale University The 4th MO, the LUMO of BH 3, is in fact an atomic 2p orbital of B. It is like the 2p SOMO that is half-occupied by the seventh electron in the analogous CH 3 radical. Note that the last three (highest energy) UMOs have nodes between B and H. This is especially clear in the 5th MO (the 3s ), which looks like a red cylinder among three blue balls. 4. Molecular orbital theory. Borane (BH3) has trigonal planar geometry ... Derive a molecular orbital diagram shown below for borane by performing the same steps as above in problem #3: (1) Determine a G describing the three H 1s atomic orbitals, (2) factor this G into a linear combination of Girr, and (3) complete the molecular orbital diagram exactly as described above for methane. Calculate your paper price Lab 5 creating walsh diagrams from computed molecular orbital ... - StuDocu Lab 5 creating walsh diagrams from computed molecular orbital energies lab report by Hanna Thomson. Course:Inorganic Chemistry (CHEM 314) Hanna Thomson. Lab 5. W ed. 4pm. Lab 5: Cr eating W alsh Diagr ams from C omput ed Molecular Orbital. Ener gies . Background . MO diagram of "B"_2"H"_6? + Example - Socratic.org For this we will get 1 + 1 + 1 + 1 + 1 +1 +1 +1 = 8. nˆR is the coefficient next to each symmetry operation (next to ˆE, ˆC2(z), ˆC2(y), etc). This is 1 in this case for all operations. Γbasis is the reducible representation. The "basis" will be either the 2s, 2px, 2py, or 2pz orbitals of boron, or the 1s orbitals of hydrogen.
5.5A: \(BH_3\) - Chemistry LibreTexts Hydrogen has higher electronegativity than boron, therefore hydrogen would have lower energy level in the MO diagram. -In addition, B has 3 electrons in the valence electrons and 3 hydrogens have total 3 electrons. Therefore, the total number of electrons filled in orbitals are 6.

0 Response to "41 molecular orbital diagram for bh3"
Post a Comment