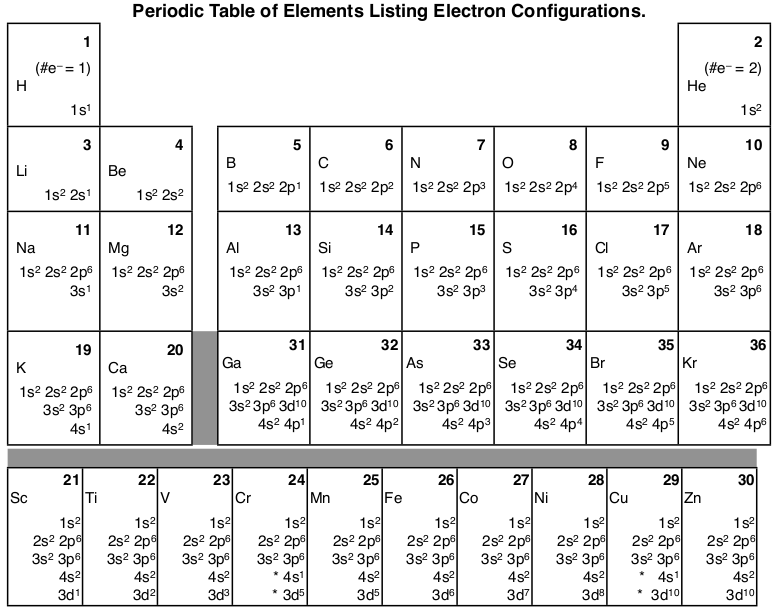
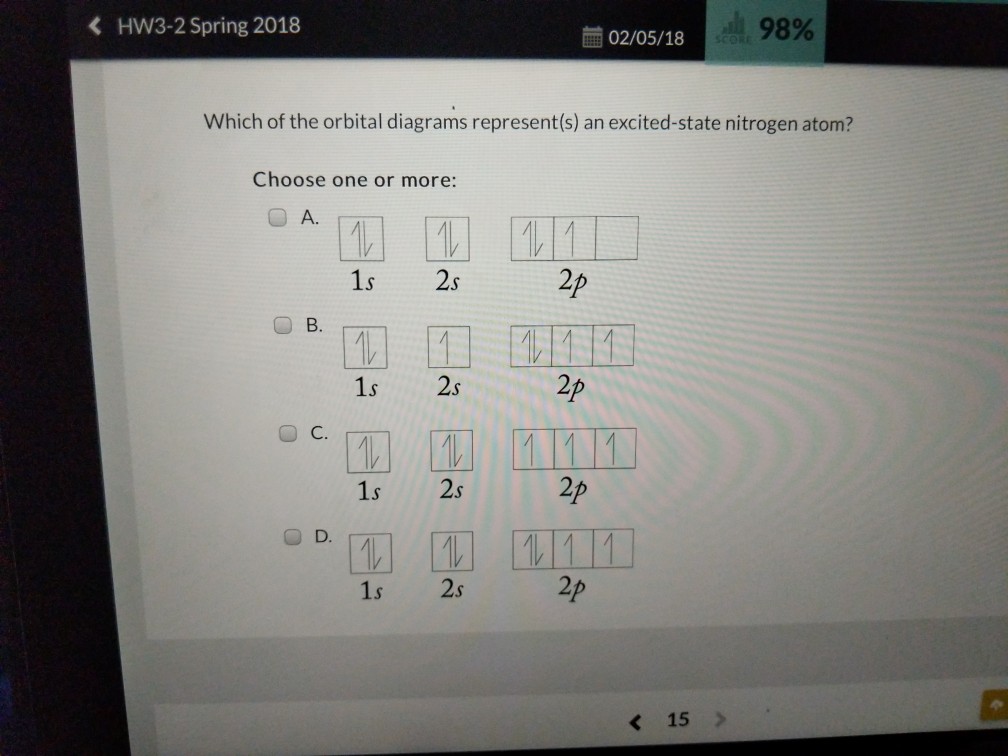
43 the orbital diagram for a ground state nitrogen atom is
Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Ground State Electron Configuration For Nitrogen When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s22s22p3. Below you can get the full image representation which will help you to understand the topic more easily. What is the orbital diagram for an atom in a ground state ... What is the orbital diagram for a ground-state nitrogen atom? Nitrogen atom has 7 electrons. The orbital diagram would look like this.^v........^v.....^v.....^ 1s2...2s2...2px..2py... What orbital...
P a g e 7 15 The orbital diagram for a ground state ... P a g e 7 15 The orbital diagram for a ground state nitrogen atom is 1s 2 2s 2 from PHYS 240 at San Francisco State University

The orbital diagram for a ground state nitrogen atom is
Which one of the following is the correct orbital diagram ... Which one of the following is the correct orbital diagram for a ground state from CHEM 2045 at Indiana University, Bloomington. Study Resources. Main Menu; ... Which one of the following is the correct orbital diagram for a ground-state nitrogen (N) atom? A) B) ... P atom is smaller than the Mg atom since P has a smaller ionization energy. B) P ... What is the orbital diagram for a ground-state nitrogen ... electron configuration. A nitrogen atom has 3 orbitals; the 1s orbital, the 2s orbital, and the 2p orbital. In this case, the 2s and 2p orbitals are the valence orbitals, as they have the electrons... CHEM Final Flashcards - Quizlet Which electron orbital diagram is written correctly for an atom without any violations? ^ ^ ^^^ Which is the correct electron configuration for a nitrogen atom? 1s22s22p3. Which is the correct electron configuration for an oxide ion? ... What is the principal quantum number for the outermost electrons in a Te atom in the ground state? 5.
The orbital diagram for a ground state nitrogen atom is. [ANSWERED] Which Of The Orbital Diagrams Represent(S) An ... Electronic configuration of nitrogen in ground state is 1s2 2s2 2p3 or 1s2 2s2 2px1 2py1 2pz1. Hence, in excited state one of the 2s electron will jump to 2p orbital,so the excited state electronic configuration should be 1s2 2s1 2px2 2py1 2pz1. Chemistry - Chapter 9 Flashcards - Quizlet The lowest energy orbital in the quantum-mechanical model is the. 1s orbital. Which orbital would the electron of a ground state hydrogen atom occupy? 1s. How many electrons are unpaired in the orbitals of carbon? 2. How many electrons are unpaired in the orbitals of nitrogen? 3 "When filling orbitals of equal energy, electrons fill them singly ... Q9 which of the following electron ... - Course Hero The electron configuration of a ground-state Cobalt atom is (Co, z - 27) A. [Ar]4 s 2 3 d 7 B. 1 s 2 2 s 2 2 p 6 3 s 2 3 d 9 C. [Ne]3 s 2 3 d 7 D. [Ar]4 s 1 3 d 5 Upload your study docs or become a Course Hero member to access this document Solved The orbital diagram for a ground-state nitrogen ... Expert Answer Option (A) is the correct orbital diagram for a ground -state nitrogen atom . Electronic configuration of nitrogen in ground state is, 1s22s22p3 or 1s22s22px12 … View the full answer Transcribed image text: The orbital diagram for a ground-state nitrogen atom is 1s 2s 2p A lt 1 Î Î Î B.1 c.lt Į I Į D실 싶 싶 소 소 A B c С O D D
22The orbital diagram for a ground state nitrogen atom is ... 22.The orbital diagram for a ground-state nitrogen atom is 1s 2s A B C D 2p A) A B) B C) C D) D 23. Which ground-state atom has an electron configuration described by the following orbital diagram? Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12 ... Ch 7/8 quiz Flashcards | Quizlet red The orbital diagram for a ground-state oxygen atom is 1s (up down) 2s (up down) 2p (up down, up, up) Which of the following is the electron configuration of an excited state of an oxygen atom? 1s^2 2s^2 2p^3 3s^1 Select True or False: An electron gains energy in the transition from a 6s subshell to a 5d subshell. true How many electrons are unpaired in the orbitals of nitrogen? 3 Unpaired electrons. Nitrogen atom has total 7 electrons. Two will fill up the n=1 level, and then there are five electrons in the n=2 level. Nitrogen can bond three times with other electrons to fill up it's shell with 8, (8-5=3). And these are those 3 unpaired electrons which were residing the 2p sub-shell of the Nitrogen atom , before the formation of 3 bonds.
Magnesium Orbital diagram, Electron configuration, and ... The orbital diagram simply represents the arrangement of electrons in the different orbital of an atom, it uses an arrow to represent the electrons, every orbital (one box) contains a maximum of 2 electrons. There are three rules followed for constructing the orbital diagram for an atom. (1). HW #3 Flashcards - Quizlet orbital node crevice pit Node The ground-state electron configuration of a calcium atom is [Ne]3s2 [Ar]4s13d1 [Ne]3s23p6 [Ar]4s2 [Ar]3d2 [Ar]4s2 Consider the element with the electron configuration [Kr]5s24d7. This element is an actinide element. a halogen. a noble gas. a transition metal. a nonmetal. a transition metal. Nitrogen Orbital diagram, Electron configuration, and ... The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows- What orbital diagram correctly represents the outermost ... Nitrogen (N) has atomic number 7, so the electron configuration is 1s2 2s2 2p3. The outermost energy level is level 2 (n=2) so there are a total of FIVE electrons in the outermost energy level.
Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state. Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub-orbital ...
Use The Orbital Filling Diagram To Show The Electron Configuration Of Phosphorus P - General ...
the orbital diagram for a ground state oxygen atom is Get the detailed answer: the orbital diagram for a ground state oxygen atom is. Get the detailed answer: the orbital diagram for a ground state oxygen atom is. 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... the orbital diagram for a ground state oxygen atom is ...
Exam 3 Study Guide Flashcards | Quizlet Recall that for hydrogen En =-2.18 × 10 ⁻¹⁸ J (1/n2) 3.08 × 10¹⁵ /s A possible set of quantum numbers for the last electron added to complete an atom of gallium (Ga) in its ground state is n l ml ms 4 1 0 +1/2 The orbital diagram for a ground-state nitrogen atom is 1s 2s 2p ↑↓ ↑↓ ↑ ↑ ↑ The orbital diagram for a ground state carbon atom is 1s 2s 2p
What is the orbital diagram for a ground state carbon atom ... The ground state is 1s^2 2s^2 2p^2. In the explanation below, I show a common means of diagramming this. Using arrows to show the spin orientation of each electron, the orbital diagram is often shown this way: The single electrons in the two p-orbitals is in accordance with Hund's Rule.
The orbital diagram for a ground-state nitrogen atom is C ... 9. a) Give the ground state electron configuration of a nitrogen atom. b) Give the ground state electron configuration of a carbon atom. c) Draw the Lewis structure for CN and include the formal charge on appropriate atom for the Lewis structure d)...
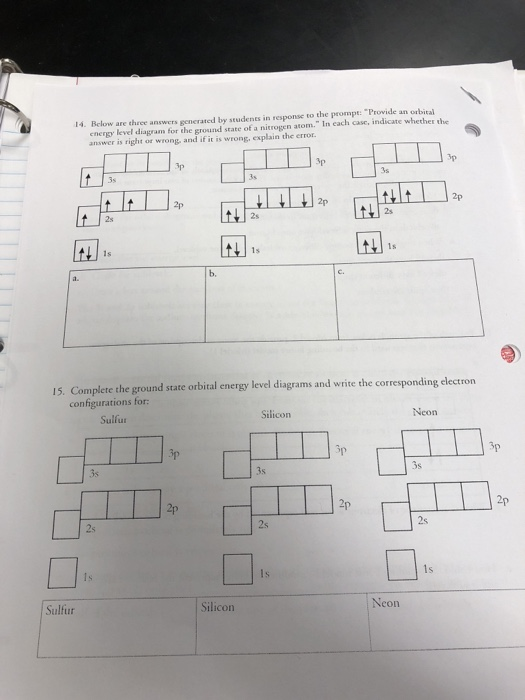
Sulfur Orbital diagram, Electron configuration, and ... The Sulfur orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the remaining four electrons in the 3p orbital. An orbital diagram for a ground-state electron configuration of a Sulfur atom is shown below-

Molecular diagram of a ground state diatomic oxygen molecule ( 3 ∑ gO 2... | Download Scientific ...
Solved The orbital diagram for a ground-state nitrogen ... The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소 ; Question: The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소
CHEM Final Flashcards - Quizlet Which electron orbital diagram is written correctly for an atom without any violations? ^ ^ ^^^ Which is the correct electron configuration for a nitrogen atom? 1s22s22p3. Which is the correct electron configuration for an oxide ion? ... What is the principal quantum number for the outermost electrons in a Te atom in the ground state? 5.


0 Response to "43 the orbital diagram for a ground state nitrogen atom is"
Post a Comment