41 bohr diagram of sulfur
PDF How to Draw Bohr Diagrams Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He Sulfur(S) electron configuration and orbital diagram Sulfur orbital diagram According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. The 1s orbital is now filled with two electrons. The next two electrons will enter the 2s orbital just like the 1s orbital.
Draw Bohr-Rutherford diagram for the sulfur-32 atom. | Quizlet Find step-by-step Biology solutions and your answer to the following textbook question: Draw Bohr-Rutherford diagram for the sulfur-32 atom..1 answer · Top answer: The image below shows the Bohr diagram for sulfur-32. The image below shows the Bohr diagram for sulfur-32. (Click to see the full solution)
Bohr diagram of sulfur
Sulfur Orbital diagram, Electron configuration, and Valence electrons The Sulfur orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the remaining four electrons in the 3p orbital. An orbital diagram for a ground-state electron configuration of a Sulfur atom is shown below- valenceelectrons.com › zinc-electron-configurationZinc(Zn) electron configuration and orbital diagram Electrons can be arranged correctly through orbits from elements 1 to 18. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of zinc through ... topblogtenz.com › bohr-model-of-carbonCarbon Bohr Model - How to draw Bohr diagram for Carbon(C) atom The Bohr Model of Carbon(C) has a nucleus that contains 6 neutrons and 6 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Carbon contains 4 electrons that also called valence electrons.
Bohr diagram of sulfur. valenceelectrons.com › oxygen-electron-configurationOxygen(O) electron configuration and orbital diagram Electrons can be arranged correctly through orbits from elements 1 to 18. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. PDF How to Draw Bohr- Rutherford Diagrams of Atoms shown in the Bohr Diagram for Sulfur (spread apart, not as a pair). 4. If the element has more than two electrons, then you need to add a 2nd energy level. The maximum number of electrons that can fit in the second energy level is EIGHT. 5. A single electron must be loaded in the 12 o'clock, then 3 o'clock, then 6 Bohr Model of the Atom - Overview and Examples - ThoughtCo The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted. Sulfur - Crystal Structure - Periodic Table Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
Bohr Diagram For Sulfur - schematron.org Sulfur - 4. Fluorine - 5. Calcium - 6. Argon - Name ANSWER KEY Period Date Bohr Model Diagrams. Use the information provided for each element to draw Bohr Model diagrams. Label how many of each there are in the nucleus (e.g. He: 2p, 2n). They should show all the electrons, 16 electrons in total. There are three shells containing the electrons. 4.2 Bohr Models of Ions and Compounds - hungrybeagle.com Simple compounds can be drawn using Bohr diagrams, but it's the electron configuration that is really important. There are different ways of drawing Bohr models depending on whether you have an ionic bond (between a metal and a non-metal) or a covalent bond (between 2 non-metals). Things you should be able to do after today: › articles › s41467/019/12851-wReversing the charge transfer between platinum and sulfur ... Oct 31, 2019 · The mesoporous S–C supports with high sulfur content of >12 wt% and high specific ... The value of isosurface is 0.003e/bohr 3. ... d Calculated free energy diagram of Pt(111), Pt ... pressbooks-dev.oer.hawaii.edu › phase-diagramsPhase Diagrams – Chemistry - University of Hawaiʻi Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific ...
Sulfur Bohr Model - How to draw Bohr diagram for Sulfur (S) atom According to the Bohr diagram of Sulfur, the outer shell is M-shell which contains six valence electrons. Properties of Sulfur It is multivalent and nonmetallic in nature. It appears as bright yellow and crystalline solid at room temperature. It has a boiling point of 444.6 °C and a melting point of 115.21 °C. Bohr Model of all Elements (Diagrams + Chart Inside) - Periodic Table Guide Bohr Model of all Elements (Diagrams + Chart) Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. Bohr Diagram Of Sulfur The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the third layer. This is because the atomic number of Potassium (K) is 19, therefore has 19 protons and 19 electrons. What is the Bohr's model for sulfur? Bohr Rutherford Diagram For Sodium In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Theoretically, they.A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps ...
techiescientist.com › h2s-lewis-structureH2S Lewis Structure, Molecular Geometry, Hybridization, and ... May 24, 2022 · H2S Molecular Orbital (MO) Diagram. The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals.
Bohr model - Wikipedia In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.
How to Draw the Bohr-Rutherford Diagram of Sulfur - YouTube Sulfur / Sulphur has 2 electrons in its first shell, 8 in its second, 6 in its third.Check me out:
PDF 8 ATOMIC NUMBER SYMBOL - cusd80.com 14. Scientists use two types of diagrams to show the electron configuration for atoms. Follow your teacher's directions to complete the diagrams. Sulfur Atomic # = 16 Atomic Mass = 32 Protons = 16 Neutrons = 16 Electron = 16 15. Calculate the missing information and then draw the Bohr Diagram and Lewis Structure for each element. Mg Atomic ...
techiescientist.com › sf6-lewis-structureSF6 Lewis Structure, Molecular Geometry, Hybridization, and ... May 24, 2022 · Molecular Geometry and MO Diagram of SF6 There are Fluorine atoms all around Sulfur which gives the compound a kind of symmetry when we look at it on a planar level. When we look at the molecular geometry of any compound we get to see a 3-D image of how the atoms are distributed which we cannot identify while making a Lewis Structure.
Sulfide | S-2 - PubChem 2022-04-23. Create. 2004-09-16. Sulfide (2-) is a divalent inorganic anion obtained by removal of both protons from hydrogen sulfide. It is a conjugate base of a hydrosulfide. Chemical groups containing the covalent sulfur bonds -S-. The sulfur atom can be bound to inorganic or organic moieties.
Bohr model of the atom - Chemistry Resource According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons.
Bohr Diagram - The Element Sulfur The Element Sulfur: All About Sulfur! Bohr Diagram; Powered by Create your own unique website with customizable templates. Get Started ...
Bohr Diagram For Sulfur Answer to Draw a Bohr model for an atom of sulfur (S). How many additional electrons can fit into the n = 3 shell?.Bohr Diagrams1) Find your element on the periodic table.2) Determine the number of electrons - it is the same as the atomic number.3) This is how many electrons you will draw. 6.
Iodine Bohr Diagram - Wiring Diagrams Iodine Bohr Diagram. Iodine 2,8,18,18,7. I. Sulfur!! Sulfur!! Atom Model Project, Mass Number, Bohr Model, Teaching Chemistry, [Bohr Model of Iodine] Bohr Model, Atomic Number, School Stuff, Health. This Pin was discovered by Mi Escuelita Montessori Montessori. Discover (and save!) your own Pins on Pinterest.
What is the Bohr diagram for sulfur? | Study.com In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3,.... The sphere n = 1 can accommodate two, the n=2-3 ...1 answer · Top answer: Sulfur has 16 electrons, 16 protons and considering the 3216S1632S isotope, 16 neutrons. Shell n=1 has 2 electrons and shells n=2 has 8...
Bohr Diagram Of Sulfur - schematron.org In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3,. The sphere n = 1 can accommodate two, the n = Model sulfur atoms are complex, containing nearly 50 parts. the Bohr atom model with fixed electrons as a way to simplify atomic structure. Sulfur (S).
Bohr Model Diagram Worksheet Answers - defenderring.co Bohr model and lewis dot diagram worksheet answers or 50 best stock lewis dot diagram for sulfur diagram insp. Change the color of the january tab to blue and the. Source: Bohr model scavenger hunt answer sheet for each problem write the name of the bohr model in the boxes below. Bohr model diagrams and lewis dot structures.
How to Draw Bohr-Rutherford Diagrams - Phosphorous - YouTube How to draw the Bohr-Rutherford Diagram for Phosphorous. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
What is the orbital diagram for sulfur? Electronic configuration of Sulfur is 2,8,6 as its atomic number is 16. So,for sulfur to attain stability it has to gain 2 electron. Therefore combining capacity of sulfur or valency is -2. Compunds ofsulphur like SO3, SO2, etc have a valency of 6.
Isotope data for sulfur-35 in the Periodic Table Isotopes of Sulfur (click to see decay chain): 26 S 27 S 28 S 29 S 30 S 31 S 32 S 33 S 34 S 35 S 36 S 37 S 38 S 39 S 40 S 41 S 42 S 43 S 44 S 45 S 46 S 47 S 48 S 49 S : 35 S : Half-life: Fermion, 16p 19n: 87.51157407407 d: Spin 3/2 Parity 1: ... Click any isotope in diagram to see its data.

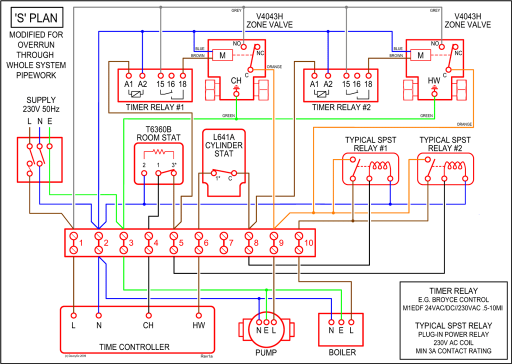







0 Response to "41 bohr diagram of sulfur"
Post a Comment