45 given the potential energy diagram for a reaction
The potential energy diagram for a reaction R → P is given below. Δ H ... The potential energy diagram for a reaction R → P is given below. ΔH ⊖ of the reaction corresponds to the energy : A x B y C z D (x = y) Medium Solution Verified by Toppr Correct option is C) From the given figure, The activation energy of the forward reaction is x. The activation energy of the backward reaction is z. and, as we know, ΔH=E b −E f Solved 5. Given the Potential Energy Diagram for a 3-step | Chegg.com 5. Given the Potential Energy Diagram for a 3-step reaction, answer the questions below: POTENTIAL ENERGY ACTION a) Which arrow indicates the activation energy for the first step of the reverse reaction? b) Which arrow indicates the activation energy for the first step of the forward reaction?
Look at the potential energy diagram for a chemical reaction. Which ... Look at the potential energy diagram for a chemical reaction. Which statement correctly describes the energy changes that occur in the forward reaction? The activation energy is 10 kJ and the reaction is exothermic. The activation energy is 10 kJ and the reaction is endothermic. The activation energy is 50 kJ and the reaction is exothermic.

Given the potential energy diagram for a reaction
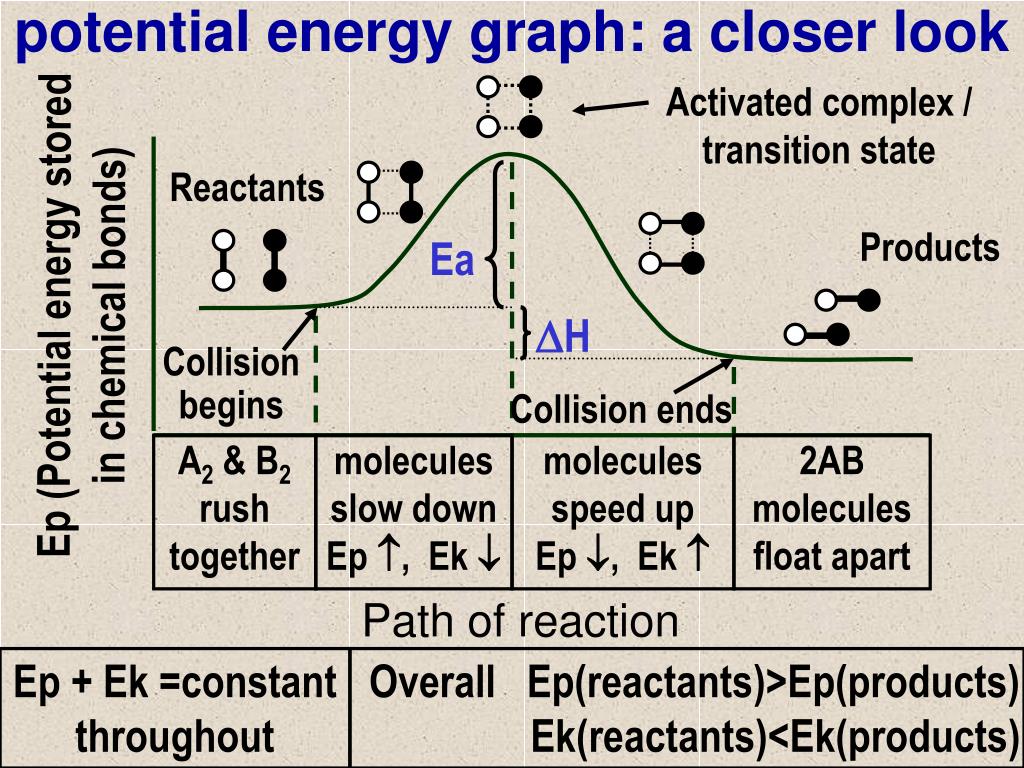
Answered: 1. Given the following potential energy… | bartleby 1. Given the following potential energy diagram for the reaction X + Y ---> Z + R The arrow "d" represents the b a x+y d z+R Reaction coordinate A) energy content of the products B) activation energy of the forward reaction C) energy content of the reactants D) activation energy for the reverse reaction E) net change/energy difference for the reaction Potential energy Potential Energy Diagrams | Chemistry for Non-Majors Figure 1. A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. PDF Representing a Reaction with a Potential Energy Diagram r based upon the given potential energy diagram and indicate if the reaction endothermic or exothermic. What Is Given? You have a potential energy diagram. Plan Your Strategy Act on Your Strategy Refer to the scale on the y-axis and determine the difference in energy between the reactants and the transition state, E a(fwd); the difference in energy
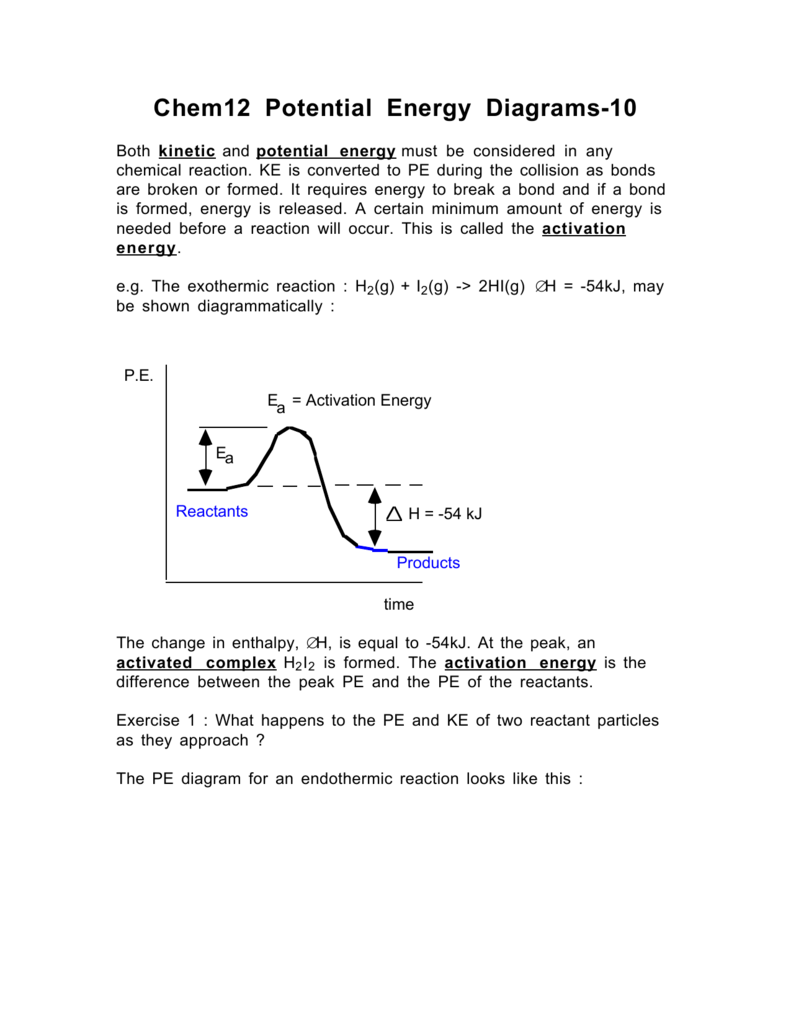
Given the potential energy diagram for a reaction. Regents Chemistry Exam Explanations June 2012 Given the potential energy diagram representing a reversible reaction; The activation energy for the reverse reaction is represented by (1) A + B (2) B + C (3) B + D (4) C + D: 2: link: go up the hill backwards. B+C: 2: link: chlorine goes on the 2nd carbon in of a 4 carbon chain. How to draw the potential energy diagram for this reaction? C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction. PDF The Leon M. Goldstein High School for the Sciences The potential energy diagram below represents a reaction. PE 40 Potential Energy Diagram pathway without with catalyst Course Ca culate the heat o reaction. What effect does a catalyst have in a ... potential energy that occur during the given reaction. Given the reaction: A + B --> C a) Does the diagram illustrate an exothermic or an ... PDF Version 1 - Garden City Public Schools / Homepage A) 1 B)2 C) 3 D) 4 25.Given the reaction: N2(g) + 2 O2(g) 2 NO2(g) H = + 7.9 kcal/mole The potential energy diagram of the reaction is shown below. Which arrow represents the heat of reaction ( H) for the reverse reaction? Version 1 A) increasing B)constant C) decreasing D) zero 26.A reaction reaches equilibrium at 100 °C.
PDF Forest Hills High School Given the potential energy diagram for a reversib chemical reaction: Reaction Coordinate Each interval on the axis labeled "Potential Energy (kJ/mol)" represents 10. kilojoules per mole. Wha is the activation energy of the forward reaction? C) D) Reaction Coordinate Reaction Coordinate Reaction Coordinate Reaction Coordinate A) 10. chemistry Test Worksheet: Heat Of Reaction Amd Potential Energy Diagram (1) A + B (2) B + C (3) B + D (4) C + D 7 Given the equation and potential energy diagram representing a reaction: If each interval on the axis labeled "Potential Energy (kJ/mol)" represents 10. kJ/mol, what is the heat of reaction? (1) +60. kJ/mol (2) +20. kJ/mol (3) +30. kJ/mol (4) +40. kJ/mol 8 Solved Given the following potential energy diagram for a ... Given the following potential energy diagram for a 3 step reaction, label or identify which arrow shows these categories for both the forward and reverse reactions (do forward in one color and reverse in a different color): a.) Reactant (s) b.) Product (s) c.) Intermediate (s) d.) Kinetics and Equilibrium TICKET Quiz - Quizizz Which information about a chemical reaction is provided by a potential energy diagram? answer choices the oxidation states of the reactants and products the average kinetic energy of the reactants and products the change in solubility of the reacting substances the energy released or absorbed during the reaction Question 15 30 seconds Q.
PDF - Mrs. Gaon's Chemistry Corner Given the potential energy diagram for a chemical reaction: Which statement correctly describes the energy changes that occur in the forward reaction? A. The activation energy is 10. kJ and the reaction is C. The activation energy is 50. kJ and the reaction is endothermic. endothermic. B. Potential Energy Diagrams - Chemistry | Socratic Explanation: the rate of a chemical reaction ( A+B= C+D) is given in first approximation by V = Kv × [A] ×[B] where Kv = K° ×e− Ea RT where K° depends by many factors as solvent, geiometry, type of reaction Ea is the activation energy R is the universal costant of gases T is the absolute temperature PEP - PER 1.) For a chemical reaction, the difference between ... Given the potential energy diagram and equation representing the reaction between substances A and D: system gained so must be endothermic. Cookin) heat nrg.4 pages Consider the potential energy diagrams for two types of ... The reaction of G2 and E2 to form 2EG is exothermic, and the reaction of G2 with X2 to form 2XG is endothermic. The activation energy of the exothermic reaction is greater than that of the endothermic reaction. Sketch the potential energy profile diagrams for these two reactions on the same graph.
The potential energy diagram for a reaction X to Y class 11 chemistry CBSE A potential energy diagram represents the change in potential energy of a system as reactants are changed into products. Complete step by step answer: We have to know that the Arrhenius equation is helpful in calculating the rate of reaction and plays an important portion in chemical kinetics. We can write Arrhenius equation as, K = A e − E a / R T
(Solved) - Use the potential energy diagram of a chemical reaction shown... (1 Answer) | Transtutors
Potential energy diagrams - Controlling the rate - Higher ... This can be shown by a potential energy diagram: E A is the activation energy (energy required to start the reaction) \(\Delta H\) is the quantity of energy given out (ie the enthalpy change) For ...
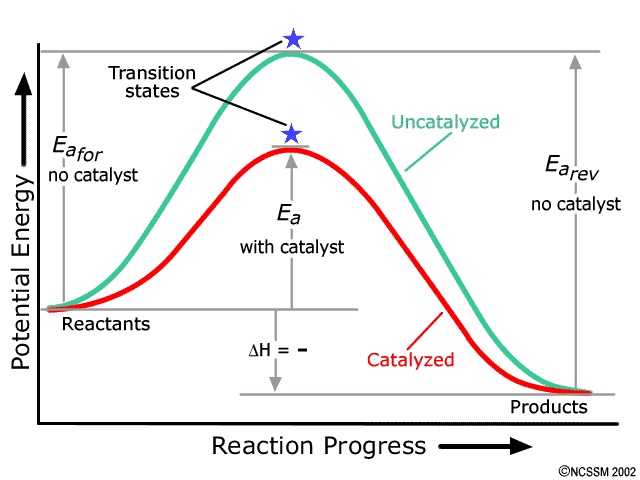
Given the potential energy diagram for a reaction: Which ... Chemistry High School answered • expert verified Given the potential energy diagram for a reaction: Which intervals are affected by the addition of a catalyst? (1) 1 and 2 (2) 1 and 3 (3) 2 and 4 (4) 3 and 4 Advertisement Expert-verified answer priyankatutor Intervals 1 and 3 are most affected by the addition of catalyst. Option 2 is correct.
PDF SPRING TERM REVIEW SHEET-2018 Name: Date - The Leon M ... The potential energy diagram for a chemical reaction is shown below. Each interval on the axis labeled fiPotential Energy (kJ)fl represents 40 kilojoules. What is the heat of reaction? A. 120kJ B. 40kJ C. +40kJ D. +160kJ page 6 SPRING TERM REVIEW SHEET-2018 25. Base your answer(s) to the following question(s) on the information below.
Answer Keus- POTENTIAL ENERGY DIAGRAM PE Diagrains. 575. 1. Given the potential energy diagram for a reaction: 4. Given the equation and potential energy diagram representing a reaction: A+B C.8 pages
The potential energy diagram for a reaction X→ Y is given ... Click here to get an answer to your question ✍️ The potential energy diagram for a reaction X→ Y is given. A and C in the graph corresponds to:1 answer · Top answer: Activation energy is defined as the least possible amount of energy (minimum) which is required to start a reaction or the amount of energy available ...
PDF Kinetics and Equilibrium Review - MS. DRURY'S CHEMISTRY ... A) 1 B) 2 C) 3 D) 4 1.Given the potential energy diagram for a reaction: Which interval on this diagram represents the difference between the potential energy of the products and the potential energy of the reactants? A) The activation energy is 10. kJ and the reaction is endothermic.

PPT - In the iodine clock reaction, there are really two processes happening simultaneously. The ...
4-29-14 PE Diagram Review Key.pdf The graph below represents the potential energy changes that occur in a chemical reaction. 4. 200+0₂ reactants. Potential Energy. ロー.4 pages
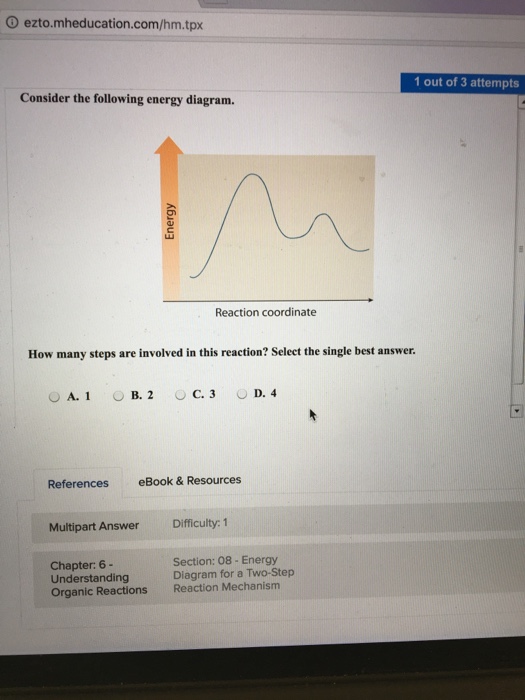
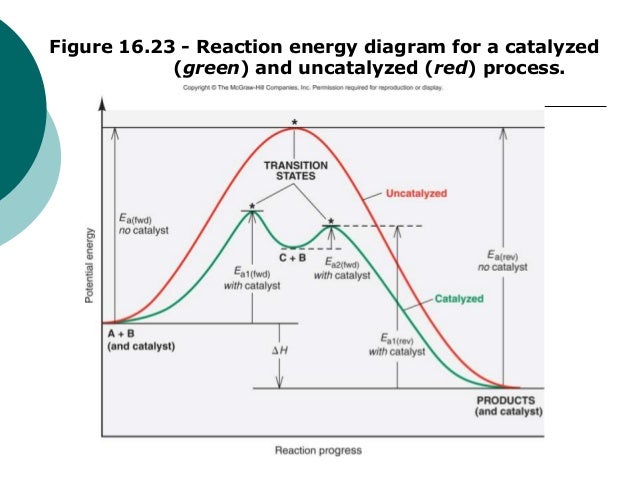
Mechanisms and Potential Energy Diagrams - Lumen Learning The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2 .Each elementary step has its own activated complex, labeled AC 1 and AC 2 .Note that the overall enthalpy change of the reaction is unaffected by the individual steps, since it depends only on the initial and final states.
6 Given the potential energy diagram for a chemical ... A) -120kJ B) +40kJ ionization energy B) heat of reaction C) activation energyC) -40kJ D) +160kJ10.The potential energy diagram for a chemical reaction is shown below. What occurs during this reaction? Each interval on the axis labeled "Potential Energy (kJ)" represents 40 kilojoules.
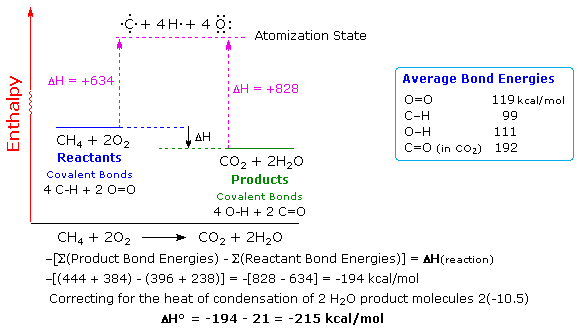
Potential Energy Diagrams - Kentchemistry.com According to Table I, which potential energy diagram best represents the reaction that forms H2O(l) from its elements? Answer--> Choice 1, since H is negative 3. Given the reaction: CH4(g) + 2 O2(g) --> 2 H2O(l) + CO2(g) What is the overall result when CH4(g) burns according to this reaction? (1) Energy is absorbed and ΔH is negative.
PDF Chemistry C T S G - nyostrander.us 3. Given the potential energy diagram for a reversible chemical reaction on the right: Each interval on the axis labeled "Potential Energy (kJ/mol)" represents 10. kilojoules per mole. What is the activation energy of the forward reaction?
Heat Of Reaction Amd Potential Energy Diagram 7 Given the potential energy diagram for a reversible chemical reaction: Each interval on the axis labeled "Potential Energy (kJ/mol)" represents 10. kilojoules per mole. What is the activation energy of the forward reaction? (1) 10. kJ/mol (3) 40. kJ/mol (2) 30. kJ/mol (4) 60. kJ/mol 8 In chemical reactions, the difference between the
PDF Potential Energy Diagram Worksheet ANSWERS Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to ... Draw an energy diagram for a reaction. Label the axis, PE of reactants = 350 KJ/mol, Ea = 100 KJ/mol, PE of products = 250 KJ/mol. 7. Is the reaction in # 6 exothermic or endothermic?
PDF Representing a Reaction with a Potential Energy Diagram r based upon the given potential energy diagram and indicate if the reaction endothermic or exothermic. What Is Given? You have a potential energy diagram. Plan Your Strategy Act on Your Strategy Refer to the scale on the y-axis and determine the difference in energy between the reactants and the transition state, E a(fwd); the difference in energy
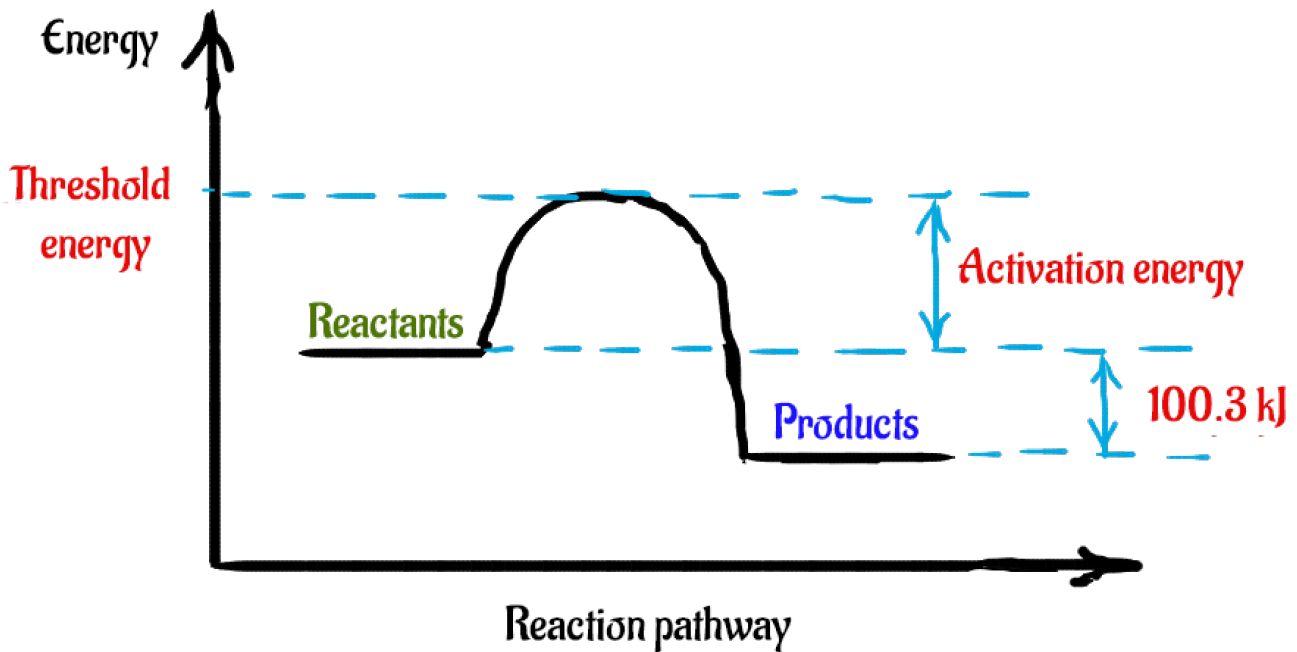
How would you draw an enthalpy diagram for: N_(2(g)) + 3H_(2(g)) -> 2NH_(3(g)) DeltaH= -"100.3 ...
Potential Energy Diagrams | Chemistry for Non-Majors Figure 1. A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive.
Answered: 1. Given the following potential energy… | bartleby 1. Given the following potential energy diagram for the reaction X + Y ---> Z + R The arrow "d" represents the b a x+y d z+R Reaction coordinate A) energy content of the products B) activation energy of the forward reaction C) energy content of the reactants D) activation energy for the reverse reaction E) net change/energy difference for the reaction Potential energy






0 Response to "45 given the potential energy diagram for a reaction"
Post a Comment