38 li2+ molecular orbital diagram
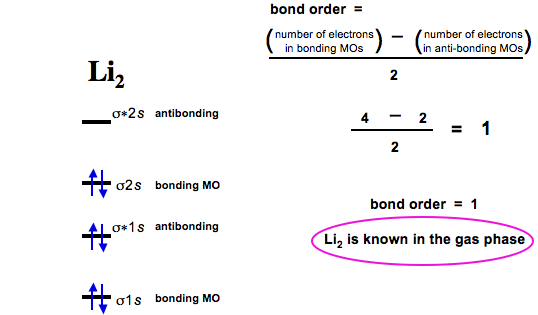
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM - Department of Chemistry orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the infinite-fold rotation axis of the orbitals on the basis of the change in wave function sign upon crossing the nodes on the bond axis. 5.10 a. OF- has 14 valence electrons, four in the π 2p* orbitals (see the diagram in the answer to Problem 5.9). b. Li2- Molecular Orbital Diagram - schematron.org The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule . Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1.
Li2 Molecular Orbital Diagram Molecular orbital energy level of Li2.This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as .

Li2+ molecular orbital diagram
Molecular Orbital Diagram For Li2 - schematron.org This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Why "Li"_2^+ is more stable than "Li"_2 ? | Socratic Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and Molecular Orbital Theory - Chemistry Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. This shows the MO diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the effective nuclear charge increases and atomic radius ...
Li2+ molecular orbital diagram. Solved Draw molecular orbital diagrams for the molecules Li2 - Chegg Draw molecular orbital diagrams for the molecules Li2 and Li2-, considering only the valence electrons.a. Identify the Highest Occupied Molecular Orbital (HOMO) for both species. b. Which would have a stronger Li-Li bond? Be sure to use your molecularorbital diagram to support your answer. Solved Construct the molecular orbital diagram for Li2. Note - Chegg Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. Li Li Li Answer Bank 2s 2s、、 02s Identify the bond order O 0 O 05 O 1 O 1S 02.5 ; Question: Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. N2 Lewis Structure, Molecular Geometry, and Hybridization When two orbitals are added, the result is stable bonding molecular orbital and when orbitals are subtracted, it is called unstable anti-molecular bonding (*) which has more energy than the latter one. Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1. Molecular Orbital Diagram Maker - University of Sydney ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
MOT | Molecular Orbital Energy level Diagram for Li2, Li2+ , Li2 ... This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o... Li2- Molecular Orbital Diagram Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. How to predict the existence of Li2 C2 with their molecular orbital ... Which is the molecular orbital diagram for HF? The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already explained ,only a pz orbital is able to combine with s orbital. Schupf Computational Chemistry Lab - Colby College The interaction of 4th lone pair donor orbital, 13, for S3 with the lone pair acceptor orbital, 9, for Li2 is 59.0 kJ/mol. Top of page. Molecular Orbital Energies The orbital energies are given in eV, where 1 eV=96.49 kJ/mol. Orbitals with very low energy are core 1s orbitals.
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Molecular Orbital Diagram For Li2 - Wiring Diagrams Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1. Molecular Orbital Diagram (MO Diagram) of Li2 - YouTube Full first shell:sigma-1s and sigma1s* are both full.sigma-2s is full; sigma-2s* is empty.Bond order 1 = single bond.Check me out: What is the bonding order of Li2? - Quora Molecular orbital electronic configuration of Li₂- : σ1s² σ*1s²σ2s2 σ*2s¹, bond order = (Nb - Na)/2 = (4-3)/2 = 0.5. As the bond orders are same in both the species, it is expected that their bond lengths are same. But as more electrons in anti bonding orbital lengthens the bond, the bond length of Li₂- is slightly greater than that of Li₂+.
how to draw molecular orbital diagram of n2 - Minta Hutton Use the molecular orbital diagram B C2 N2 O2 A. Molecular orbital electron configuration diagram for Li2 Figure Molecular orbital. Use the molecular orbital diagram to figure out the electronic configuration for N2. Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules.
F2 Lewis Structure, Molecular Geometry, Hybridization ... - Techiescientist Fluorine, with the chemical formula F2, is a pale yellow-colored diatomic gas, which has a pungent odor. F2 has a molecular weight of 37.997 g/mol. Its boiling point is −188 °C, and its melting point is −219.67 °C. It is toxic in nature; it can cause chemical burns on the skin and can be lethal if inhaled. It is highly reactive, is ...
Molecular Orbitals - Introductory Chemistry - 1st Canadian Edition Let's follow these guidelines and generate a molecular orbital electron configuration diagram for Li 2 (Figure 9.21 "Molecular orbital electron configuration energy diagram for dilithium"): Figure 9.21 "Molecular orbital electron configuration energy diagram for dilithium."
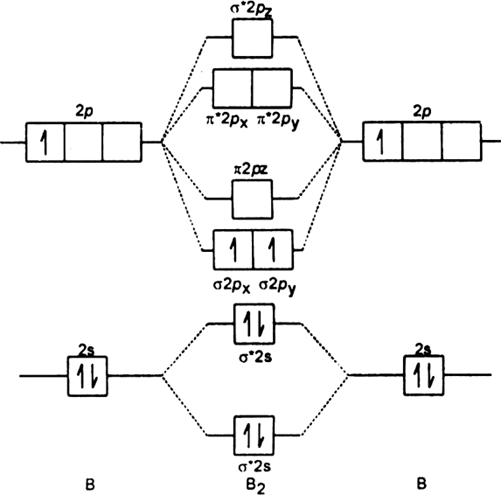
Energy level diagram for Molecular orbitals - Class Notes Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s) <σ∗(1s) < σ (2s) <σ∗(2s) < π (2px) = π (2py) < σ (2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour
Li2 Molecular orbital diagram - Brainly.in vamsheejaiswal. Ambitious. 34 answers. 4K people helped. its the MOT diagram for li2. search. rotate. arrenhasyd and 12 more users found this answer helpful. heart outlined.
Explain formation of Li2 molecule using molecular orbital diagram Consider the MO diagram for Li2. ... The electrons from the atomic orbitals forming the molecular orbitals must be filled in according to the rules mentioned previously. One electron from each atom goes down to filling the bonding orbital first, and then extra electrons would fill the antibonding orbital
Write the electronic configuration of Lithium (Li2) molecule. What is ... The number of electrons in antibonding molecular orbitals is two less than in bonding molecule orbitals. Medium. View solution. >. Ground state electronic configuration of valence shell electrons in nitrogen molecule ( N 2 ) is written as ( S 2 s ) 2 ( S ∗ 2 s ) 2 ( P 2 p ) 4 ( S 2 p ) 2 Hence, the bond order of nitrogen molecule is: Medium.
Molecular Orbital Theory - Chemistry Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. This shows the MO diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the effective nuclear charge increases and atomic radius ...
Why "Li"_2^+ is more stable than "Li"_2 ? | Socratic Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and
Molecular Orbital Diagram For Li2 - schematron.org This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.










0 Response to "38 li2+ molecular orbital diagram"
Post a Comment