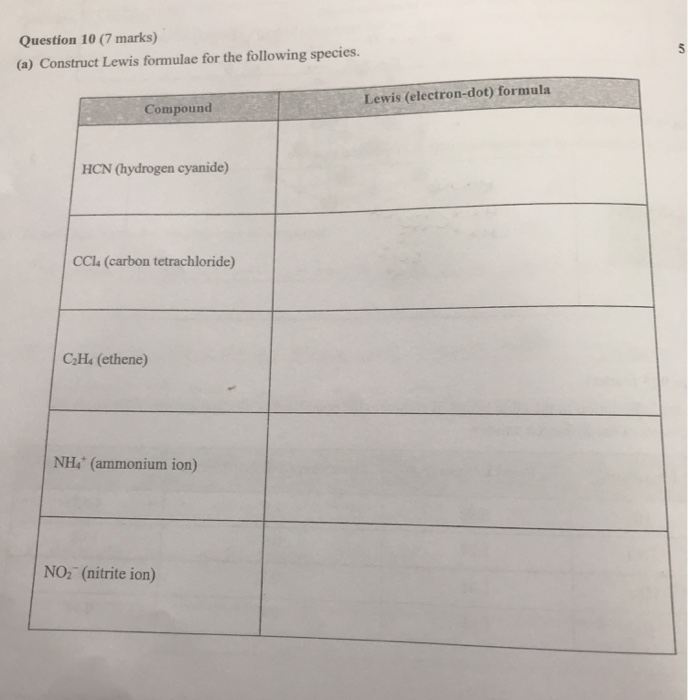
38 lewis dot diagram for c2h4
Lewis Dot Diagram For C2h4 - schematron.org Step-by-step tutorial for drawing the Lewis Structure for C2H4. The general rules for drawing electron dot diagrams are as follows; 1) Draw electron dot diagrams for each of the atoms present. 2) Draw the.The Lewis dot diagram of CH2CHCN should be simple to draw from thisstructural formula. What is the Lewis Dot Diagram for C2H4? - Answers What does the Lewis dot structure of c2h4? ... Refer to the related link for an illustration of the Lewis dot diagram for carbon dioxide. People also asked. Study Guides . Chemistry.
Lewis Dot Diagram For C2h4 - wiringall.com Lewis Dot Diagram For C2h4 The valence shell electron pair repulsion model assumes that electron pairs repel one another. Thisproduces a set of molecular geometries. Watch the video of Dr. B. drawing the Lewis dot structure for C2H4 (ethene) and answer the questions below. Note that the C2H4 Lewis dot structure involves.
Lewis dot diagram for c2h4
C2h4 Lewis Dot Structure - How to draw the lewis structure for c2h4 It's C2H4, and we want to write the dot structures for ethene. To do that, we always count our valence electrons up first. Let's take a look: Carbon is in group 4, sometimes written 14, so it has 4 valence electrons. If we come way over here to Hydrogen, it's in group 1: it has 1 valence electron. So 4 for the Carbon, but there's two of them so ... C2H4 Lewis Structure - Learnool C2H4 Lewis Structure. March 10, 2022 March 2, 2022 by Admin. C 2 H 4 (ethylene or ethene) has two carbon atoms and four hydrogen atoms. In the lewis structure of C 2 H 4, there is a double bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair. Lewis Dot Structure for C2H4 | Chemical Bonding | Success in Chemistry If we come way over here to Hydrogen, it's in group 1: it has 1 valence electron. So 4 for the Carbon, but there's two of them so we multiply it times 2. Plus one for the Hydrogen, but there's four of those so we multiply that times 4. So 2 tiems 4 is 8, plus 4 is 12. We have a total of 12 valence electrons here.
Lewis dot diagram for c2h4. C2H4 Lewis Dot Structure - How to Draw the Lewis Structure for C2H4 ----- Steps to Write Lewis Structure for compounds like C2H4 ----- 1. Find the total valence electrons for the C2H4 molecule. 2. Put the least electronegative atom in the center. Note: Hydrogen (H)... Lewis Dot Diagram For C2h2 Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. Viewing Notes: With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms. Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure. Ethene (C2H4) lewis dot structure, molecular geometry, polar or non ... As per the C2H4 Lewis structure, Four C-H sigma bonds are present and one C=C double bond (1 sigma + 1 pie bond). It means four C-H bond has 8 shared pairs of electrons and the C=C bond has 4 shared pairs of electrons. Hence total shared pairs of electrons in the dot diagram of C2H4 is 12. Why C2H4 lewis structure is planar and C2H6 is non-planar? Lewis Structure for C2H4 - UMD Drawing the Lewis structure for C 2 H 4 (named ethene) requires the use of a double bond. In a double bond two pairs of valence electrons are shared (for a total of four valence electrons). Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
C2H4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram C2H4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Hydrocarbons form an essential and inseparable portion of the science of chemistry. Be it petroleum, crude oil, or natural gas, the majority of hydrocarbons are found naturally in these fossil fuels. What is the lewis dot structure for C2H4? - Answers What is the lewis dot structure for C2H4? The structure would be C with a double bond to C. Each one of the C's have two dots on the top and bottom with a H bond on the end. So there would be 4 H's. Solved Draw the Lewis dot structure for C2H4. Include all | Chegg.com See the answer. See the answer See the answer done loading. Draw the Lewis dot structure for C2H4. Include all hydrogen atoms and nonbonding electrons. Expert Answer. What is the Lewis dot structure of C2H4? - Brainly.in What is the Lewis dot structure of C2H4? 2 See answers Advertisement Advertisement rupeshkumansi rupeshkumansi Answer: carbon and hydrogen hydrogen. Advertisement Advertisement Manvie Manvie Explanation: The Lewis structure of ammonia, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of ...
Draw Lewis electron dot structure of C2H4 - Chemistry - Shaalaa Draw Lewis electron dot structure of C2H4 . Maharashtra State Board HSC Science (General) 11th. Textbook Solutions 8018. Important Solutions 19. Question Bank Solutions 5545. Concept Notes & Videos 421. Syllabus. Advertisement Remove all ads. Draw Lewis electron dot structure of C2H4 - Chemistry ... C2H4 Lewis structure, Molecular geometry, Hybridization, Polar or ... C2H4 Lewis structure, Molecular geometry, Hybridization, Polar or Nonpolar. Leave a Comment / Chemistry / By Admin. Ethylene or ethene (C 2 H 4) Introduction. Ethylene is the chemical compound with the molecular formula C 2 H 4. It is a colorless, sweet odor gas that can be produced in nature as well as in man-made processes. draw the lewis dot structure of C2H4,C2H2 and CO2 - Brainly.in The following step can be used to draw an electron-dot structure for a compound: Count all the valence electrons present in the given molecule. Determine the central atom. (Atom with less number). Draw bonds (2 electrons) between central and the surrounding atoms. Place the remaining valence electron on the atoms as lone pair. C2H4 Lewis Structure, Molecular Structure, Hybridization, Bond Angle ... The chemical formula C2H4 represents Ethylene. This molecule is also represented by H2C=CH2, clearly showing the alkene nature of the compound. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C2H4 exists as a colorless gas and is flammable. Its odor is described as faintly 'sweet and musky'.
C2h4 Dot Structure - write the electron dot structure for ethene ... C2h4 Dot Structure - 15 images - zb 2418 dot diagram of c2h4 download diagram, lewis electron dot structures ck 12 foundation, solved draw the lewis structures for the resonance forms, hd 9767 dot diagram of c2h4 download diagram,
C2H4 Lewis Structure||How do you draw the Lewis structure for C2H4? C2H4 Lewis Structure||How do you draw the Lewis structure for C2H4?Description:To draw the C2H4 Lewis structure,you need to find out the valence electrons in...
Solved C2H Draw the Lewis dot structure for C2H4. Include - Chegg Expert Answer Transcribed image text: C2H Draw the Lewis dot structure for C2H4. Include all lone pairs of electrons. ? mil . + C H O N S P F Br Х More Part C HCO3 Draw the Lewis dot structure for H2CO3. Include all lone pairs of electrons. Q? + H O N S P F Br Part D C2N2 Draw the Lewis dot structure for C2N2. Include all lone pairs of electrons.
C2h4 Dot Diagram - Wiring Diagrams C2h4 Dot Diagram The valence shell electron pair repulsion model assumes that electron pairs repel one another. Thisproduces a set of molecular geometries. The general rules for drawing electron dot diagrams are as follows; 1) Draw electron dot diagrams for each of the atoms present. 2) Draw the. Draw the dot structure of C2H4.
Dot Diagram For C2h4 - schematron.org Answer: Answered by Expert.The Lewis structure for C2H4 consists of two "C" symbols connected by two lines; each "C" symbol is connected to two "H" symbols, with a single line for each. "C" represents carbon atoms, and "H" represents hydrogen atoms. (d) The Lewis electron-dot diagram for C2H4 is shown below in the box on the left.
Lewis Dot Diagram For C2h6 Since C has 4 valence electrons, and each H atoms contributes 1 valence electron, the total number of electrons will be 2*4 + 6*1 = 14 "e"^ (-) This means that the Lewis dot structure for C_2H_6 must account for 14 valence electrons, either through.
Lewis Dot Structure for C2H4 | Chemical Bonding | Success in Chemistry If we come way over here to Hydrogen, it's in group 1: it has 1 valence electron. So 4 for the Carbon, but there's two of them so we multiply it times 2. Plus one for the Hydrogen, but there's four of those so we multiply that times 4. So 2 tiems 4 is 8, plus 4 is 12. We have a total of 12 valence electrons here.
C2H4 Lewis Structure - Learnool C2H4 Lewis Structure. March 10, 2022 March 2, 2022 by Admin. C 2 H 4 (ethylene or ethene) has two carbon atoms and four hydrogen atoms. In the lewis structure of C 2 H 4, there is a double bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair.
C2h4 Lewis Dot Structure - How to draw the lewis structure for c2h4 It's C2H4, and we want to write the dot structures for ethene. To do that, we always count our valence electrons up first. Let's take a look: Carbon is in group 4, sometimes written 14, so it has 4 valence electrons. If we come way over here to Hydrogen, it's in group 1: it has 1 valence electron. So 4 for the Carbon, but there's two of them so ...









0 Response to "38 lewis dot diagram for c2h4"
Post a Comment