42 use the molecular orbital diagram shown to determine which of the following is most stable
chemistry 1a Chapter 10 Flashcards | Quizlet Electron groups around the central atom will be most stable when they are as far apart as possible. 2 electron groups have.... groups around the central atom, occupy positions oppiste from each other linear geometry. The bond angle is 180°. 3 electron groups have..... Answered: Use the molecular orbital diagram shown… | bartleby Solution for Use the molecular orbital diagram shown to determine which of the following is least stable. A)B2+ B)N22+ C) C22- D)B2 E) C22+.1 answer · Top answer: Step 1 ...
C22- Molecular Orbital Diagram Solution: Use the molecular orbital diagram shown to determine which of the following is most stable. A) N22+ B) B2 C) B22+ D) C E) C22+. Sketch the molecular orbital energy level diagram for the ion. How many net σ and π bonds does the ion have? What is the carbon-carbon bond order? How has the bond order changed on adding electrons to C 2 ...

Use the molecular orbital diagram shown to determine which of the following is most stable
Chem Flashcards - Quizlet Use molecular orbital diagram shown to determine which is most stable a) O22-b)F2 c) F22+ d) F22-e) Ne22-a. Use the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ ... Consider the molecule below Determine the molecular ... 66) How many of the following molecules have sp 3 hybridization on the central atom? XeCl 4 CH 4 SF 4 C 2 H 2 67). Use the molecular orbital diagram shown to determine which of the following is most stable. [ANSWERED] Use The Molecular Orbital Diagram Shown To ... Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable. Use the molecular orbital diagram shown to determine which of the following is most stable. A. F2 2 + B. Ne2 2 + C. F2 2 - D. O2 2 + E. F2. Answer
Use the molecular orbital diagram shown to determine which of the following is most stable. Answered: 1 -Use the molecular orbital diagram… | bartleby 1 -Use the molecular orbital diagram shown to determine which of the following are paramagnetic Question None Transcribed Image Text: 1 -Use the molecular orbital diagram shown to determine which of the following are paramagnetic a) F,2" b) Nez2* c) 0,2 d) 0,2 a O b O c O d O Expert Solution Want to see the full answer? Check out a sample Q&A here C22- Molecular Orbital Diagram - schematron.org It is stable. In fact, it's the perioxide ion. Check me out. The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add an electron to that diagram. This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only. The molecular ... Solved 15.) Use the molecular orbital diagram shown to ... Question: 15.) Use the molecular orbital diagram shown to determine which of the following is most stable. Atomic orbitals Molecular orbitals orbitals einding -anti s尋 2p 2p Z t Pzp It 2s 1レ 2s B2, C2, N2 b. N22 2- d. C2 e. This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (1 rating) PDF DO NOT OPEN - MiraCosta College orbitals in the set. B) A bond order of 0 represents a stable chemical bond. C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals.
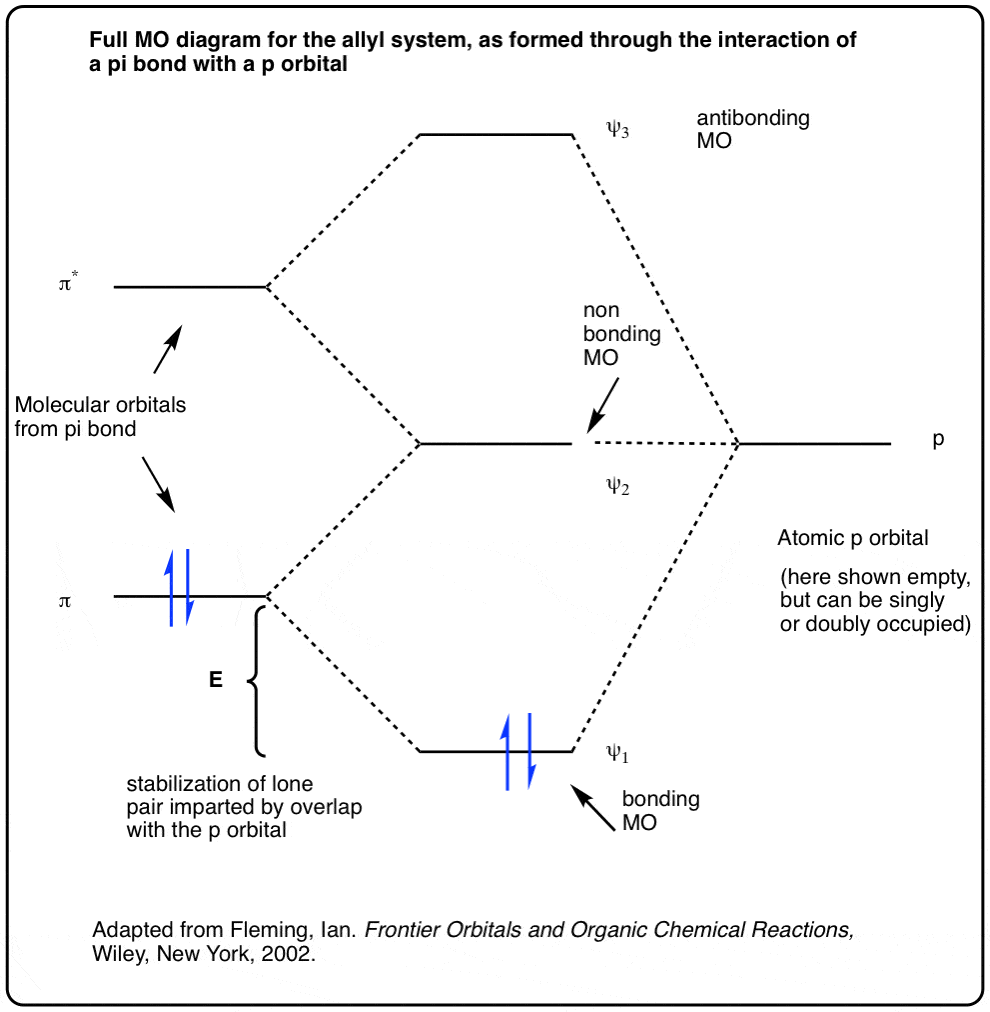
Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages Recitation Week 10 (test 3 - Recitation 2) Oct 24, 2016 1) Draw the molecular orbital diagrams to determine which of the following is most stable. A) F2 B) F2^2+ C) Ne2^2+ D) O2^2+ E) F2^2- 2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2- B) Ne2^2+ C) O2^2+ D) F2^2+ bond - Molecular orbital theory & predicting the stability ... Eg: H + H two 1s orbitals mix to form sigma and sigma*. Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding orbitals, the compound is stable. Eg: He + He; same mixing as above. Four electrons, two in the sigma, two in the sigma*. Since there are as many bonding electrons as as antibonding, there is ... 8.4 Molecular Orbital Theory - Chemistry Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... Mock Final - GitHub Pages 1) A new compound was recently discovered and found to have an atomic weight of 342.38 amu. This element has two isotopes, the lighter of which has a mass of 340.91 amu and an abundance of 68.322%. What is the mass of the heavier isotope? A) 350.21 B) 345.55 C) 342.38 D) 348.67 E) 343.29 2) Identify the characteristics of a liquid.
Molecular Orbital Theory - Chemistry 2e Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. Use the molecular orbital diagram shown to ... - Trustsu 25 Mar 2022 — Use the molecular orbital diagram shown to determine which of the following is most stable. A…. The following solution is suggested to ...1 answer · Top answer: n n Concepts and Reasonn nn n The bond order of a molecule is the number of bonds between the pair of atoms.n n n The measurement of bond order is ... Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. Chemistry - Homework AZ [ANSWERED] Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable Categories Chemistry [ANSWERED] Add Formal Charges To Each Resonance Form Of Hcno
Answered: 3. Use molecular orbital theory and the… | bartleby 3. Use molecular orbital theory and the diagram below to predict which species has the strongest bond (i.e. is the most stable). Atomic orbitals Atomic Molecular orbitals orbitals 2p 2p Energy Page 3 Name a) F2* b) F2 c) F2 d) All bonds are equivalent according to molecular orbital theory. Question Please refer to the photo! Thank you so much!
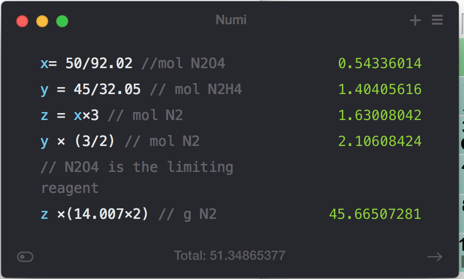
Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable ...
[Answered] Use the molecular orbital diagram shown to ... The theory uses Molecular Orbital (MO) diagram to explain the bonding between atoms. For example, to determine the stability of an atom, bond order is calculated. Bond order is calculated to determine the strength and length of the bond. The high value of the bond order means the bond is shorter, stronger, and have high bond strength.
Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable ...
Use the molecular orbital diagram shown to determine which ... A molecule is stable when bond order is high. Bond order is high when more electron is in bonding molecular orbital. O 2 2+ has only 10 electrons, so the most electrons are in bonding molecular orbital with bond order 3. So, the molecule is most stable.

Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable ...
Use the molecular orbital diagram shown to determine ... Question: Use the molecular orbital diagram shown to determine which of the following is most stable. Atomic orbitals Molecular orbitals Atomic orbitals 7 Sp 2p ...1 answer · Top answer: Species having maximum BMO...

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Are Paramagnetic ...
Solved Use the molecular orbital diagram shown to ... Question: Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order. Atomic orbitals Molecular orbitals Atomic orbitals O, F, Ne Ne22 F₂2. F2 . 022- • F22 This problem has been solved! See the answer show with all required work Show transcribed image text Expert Answer 94% (31 ratings)
[ANSWERED] Use The Molecular Orbital Diagram Shown To ... Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable. Use the molecular orbital diagram shown to determine which of the following is most stable. A. F2 2 + B. Ne2 2 + C. F2 2 - D. O2 2 + E. F2. Answer

Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable ...
Consider the molecule below Determine the molecular ... 66) How many of the following molecules have sp 3 hybridization on the central atom? XeCl 4 CH 4 SF 4 C 2 H 2 67). Use the molecular orbital diagram shown to determine which of the following is most stable.
Chem Flashcards - Quizlet Use molecular orbital diagram shown to determine which is most stable a) O22-b)F2 c) F22+ d) F22-e) Ne22-a. Use the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ ...

0 Response to "42 use the molecular orbital diagram shown to determine which of the following is most stable"
Post a Comment