43 democritus atom model diagram
5 Different Atomic Models- Theories, Diagram & Structure of Atom Dalton's Model of the Atom Dalton gave a very famous theory, which stated: The matter is composed of very small particles known as atoms. Atoms are indivisible (That cannot be divided) and cannot be destroyed through chemical reactions. All atoms of an element have identical chemical properties and mass. PDF Democritus - jdenuno Democritus to Rutherford 10/6/2007 Atomic Models 2 10/6/2007 Atomic Models 3 10/6/2007 Atomic Models 4 Democritus (460— 370 BC) • Greek Philosopher • Atomism • Nothing ex ists but atoms and em pty spa ce; everyt hing els e is o pinion. • Atom comes from atomos meaning uncut table.
Chemistry, more like cheMYSTERY to me! - Democritus | The Model So Far... Exploding Can Demo The exploding can demonstration helps establish the practice of drawing particle diagrams. Students are asked to draw a particle diagram before the can is lit, while the can it lit, and when the can explodes. They come up with all sorts of explanations with their particle diagrams. Sometimes they are dead on, sometimes not.
Democritus atom model diagram
Democritus Model | Explained What is the democritus atom model? In democritus model, atoms exist not only in matter, but also in properties such as perception and the human soul. Differences in atomic shape and size determine different properties of matter. Changes in matter are the result of dissociation or combination of atoms as they move through the void. Democritus - The Atomic Model Democritus created the theory of the atom and he concluded that all mater is made up of the invisible particles called atoms. He also said that matter cannot come from nothing and matter is a "combination and recombination" of atoms. Democritus also stated that atoms could be combined to make the different matters of life. 5 Democritus Theory of Atoms - Structure - Model - AZ Chemistry In Democritus theory of atoms we can learn that the matter consists of atoms, the invisible parts, and the empty space or void. Democritus mentioned that atoms can not be destructed nor changed. He also stated that every atom is similar to each other which means that atom has no internal structure.
Democritus atom model diagram. webzoom.freewebs.com › thmsadaqagroup › FTSOnline End9th Grade Physical Science Final Exam - Webs 25. Bohr’s changes in Rutherford’s model of the atom involved the a. structure of the nucleus b. number of particles c. electrical charges of particles d. motion of electrons 26. Describe energy levels of electrons. 27. Look at the diagram. Then read the questions that follow. Choose the term or phrase that best Democritus' Idea of the Atom | Chemistry for Non-Majors | Course Hero The atomists of the time (Democritus being one of the leading atomists) believed there were two realities that made up the physical world: atoms and void. There was an infinite number of atoms, but different types of atoms had different sizes and shapes. The void was the empty space in which the atoms moved and collided with one another. Democritus Atomic Model | What was Democritus Atomic Theory? | Study.com Below are the four principles or claims that compose Democritus' atomic theory: 1) All things are composed of the atomos or the fundamental particles, atoms. 2) Atoms cannot be destroyed. 3) Atoms... Basic Model of the Atom - Atomic Theory - ThoughtCo The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus. Electrons are attracted to the protons in the nucleus, but are moving so quickly they fall toward it (orbit) rather than stick to protons.
Democritus - The History of the Atom The History of the Atom › 82128 › parts-of-an-atomWhat Are The Parts Of An Atom? - Universe Today Dec 15, 2015 · In accordance with the Standard Model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a “cloud”. Neils Bohr’s model a nitrogen atom ... Democritus atom diagram? - Answers Democritus atom diagram. Wiki User. ∙ 2008-10-10 16:10:25. See answer (1) Best Answer. Copy. if i knew i wouldn't be in here trying to look for an answer or whatever that is, don't you think im ... sciencestruck.com › labeled-atom-diagramThe Structure of an Atom Explained With a Labeled Diagram The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. This conclusion helped him propose ‘Rutherford’s Atomic Model’. According to his atom diagram, the atom has a small, positively charged nucleus in center. This nucleus carries the entire mass of the atom.
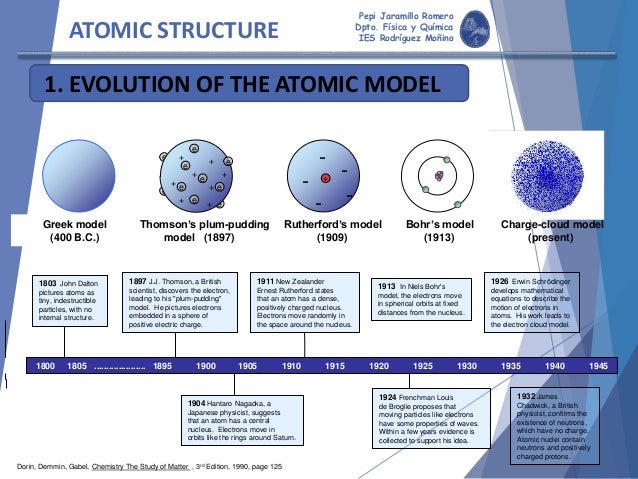
Atomic Model: Rutherford, Bohr, Dalton, Thomson - Embibe Thomson's Atomic Model J.J Thomson made the first attempt at explaining the fine structure of the atom. He proposed that-The atoms were uniform, positively charged spheres. Along with all the positive charges, most of the mass of the atom was thought to be uniformly distributed in this uniform 'cloud' of positive charge. Practice Exam Questions › uploads › 4/1/0Grade 9 Science Unit 1: Atoms, Elements, and Compounds INSIDE THE ATOM • An atom is the smallest particle of an element that retains the properties of the element. • All atoms are made up of three kinds of smaller particles call subatomic particles. • Three Subatomic Particles: Protons (positively charged) Electrons (negatively charged) Neutrons (no charge) Developing the atom - Models of the atom - AQA - GCSE Combined Science ... comes from the word 'atomos', which means uncuttable. The plum pudding model. After discovering the electron in 1897, J J Thomson proposed that the atom looked like a plum pudding. PDF Atom Democritus How has the Rutherford time? Electron Cloud Model Nuclear Atomic Model We know today that the atom is made up of 2 parts/sections: (1) The Nucleus --- (in the center of the atom) (2) The Electron Cloud --- (surrounds the nucleus) The nucleus was Discovered by Ernest Rutherford in 1911.
7 Advantages and Disadvantages of Dalton's Atomic Theory Dalton Atomic Theory. According to John Dalton the atom is like a solid ball. Dalton states that the substance consists of atoms that can not be divided again. The explanation of Dalton's theory is as follows. The element consists of particles that can not be divided again. Atoms in chemical reactions can not be changed into other elements.
Difference Between Democritus and Dalton Atomic Theory Democritus atomic theory is the ancient theory that describes the nature of matter in terms of atoms. According to Democritus (99-55 BC), atoms are infinite in number and eternal. Figure 01: Democritus We cannot create them, and the composition of atoms in a substance determines the qualities of that substance.
The Structure of an Atom: Model, Diagram, Examples - Embibe Democritus came up with the concept that matter is composed of atoms. John Dalton, in 1800 proposed the first scientific theory of atomic structure. In the article. We will learn the structure of an atom, subatomic particle, discovery, properties with some examples. Study Atoms and Molecules Concept Here. What is an Atom?
Models of the atom from Democritus to Rutherford - TKI The atom is composed of many different kinds of sub-atomic particles which are arranged in particular ways. Scientific knowledge about the atom has evolved as new evidence has been discovered. The discovery of sub-atomic particles changed how scientists view the atom.
democritus atomic model Flashcards and Study Sets | Quizlet Learn democritus atomic model with free interactive flashcards. Choose from 500 different sets of democritus atomic model flashcards on Quizlet. ... Study sets. Diagrams. Classes. Users Options. 6 terms. arianexcc. Early models of the atom: Democritus and Dalton's theory. Who was Democritus? Meaning atom. Dalton's atomic theory A. Dalton's ...
BEST TEACHERS' PAGES/LIBRARY PROJECTS: EARLY MODELS OF THE ATOM - SCAVENGER HUNT ( CHEM 11 - MS ...
thehistoryoftheatom.weebly.com › niels-bohrNiels Bohr - The History of the Atom The energy difference between the initial and final orbit is emitted by the atom in bundles of electromagnetic radiation called photons. This model was proposed in 1913 by Niels Bohr and was really an expansion on the Rutherford model of 1911. The Rutherford model had several flaws that the Bohr model overcame.
Models of the Atom Early Greek Theories Democritus Models of the Atom Early Greek Theories Democritus • 400 B. C. - Democritus thought matter could not be divided indefinitely. • This led to the idea of atoms in a void. fire earth Aristotle air water • 350 B. C - Aristotle modified an earlier theory that matter was made of four "elements": earth, fire, water, air. • Aristotle was wrong.
Democritus Atom Model Diagram - Wiring Diagrams Democritus Atom Model Diagram Democritus Atom Model Diagram Democritus was an Ancient Greek pre-Socratic philosopher primarily remembered today for his formulation of an atomic. This is due to his theory of universe that is made up of tiny "atoms", which bears .. Democritus' model of an atom was one of an intert solid that.


0 Response to "43 democritus atom model diagram"
Post a Comment