45 orbital diagram for carbon
Orbital Diagram For Carbon (C) | Carbon Electron Configuration If we give you a brief about the details, then the first two electrons will be in the 1s orbit, and following the next will be two electrons in the 2nd orbit, and the ones which are left behind, will be counted in the 2p orbital. Electron Configuration of Carbon What is the orbital notation of carbon? - FindAnyAnswer.com 4.4/5 (2,193 Views . 45 Votes) Carbon has 6 protons and electrons, so it has 2 in the 1S orbital, 2 in the 2S orbital, and 2 in the 1P orbital. This is often expressed as [HE]2S2 2Ps, because it has the same configuration as helium plus 4 additional electrons whose positions are shown after the bracketed element. Click to see full answer.
What is the orbital diagram for carbon? - Answers The orbital diagram can be derived from the elemental carbon's (C) electron (e-) configuration. C is configured as a helium (He) core as [He]2s^2 2p^2, 2, 4. A Lewis dot structure would have a C...
Orbital diagram for carbon
How do you write the orbital diagram for carbon? | Socratic The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is 1s22s22p2. The orbital diagram shows how the electrons are arranged within each sublevel. Carbon(C) electron configuration and orbital diagram Orbital Diagram for Carbon Electron configuration of carbon in the excited state Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Carbon Oxides - University of Illinois Urbana-Champaign Carbon dioxide is electron-poor at the central carbon and acts as an electrophile. The molecular orbital diagram of carbon monoxide is very similar to that of molecular nitrogen. Carbon, with 4 valence electrons, and oxygen with 6 valence electrons, together have the same number of electrons as dinitrogen.
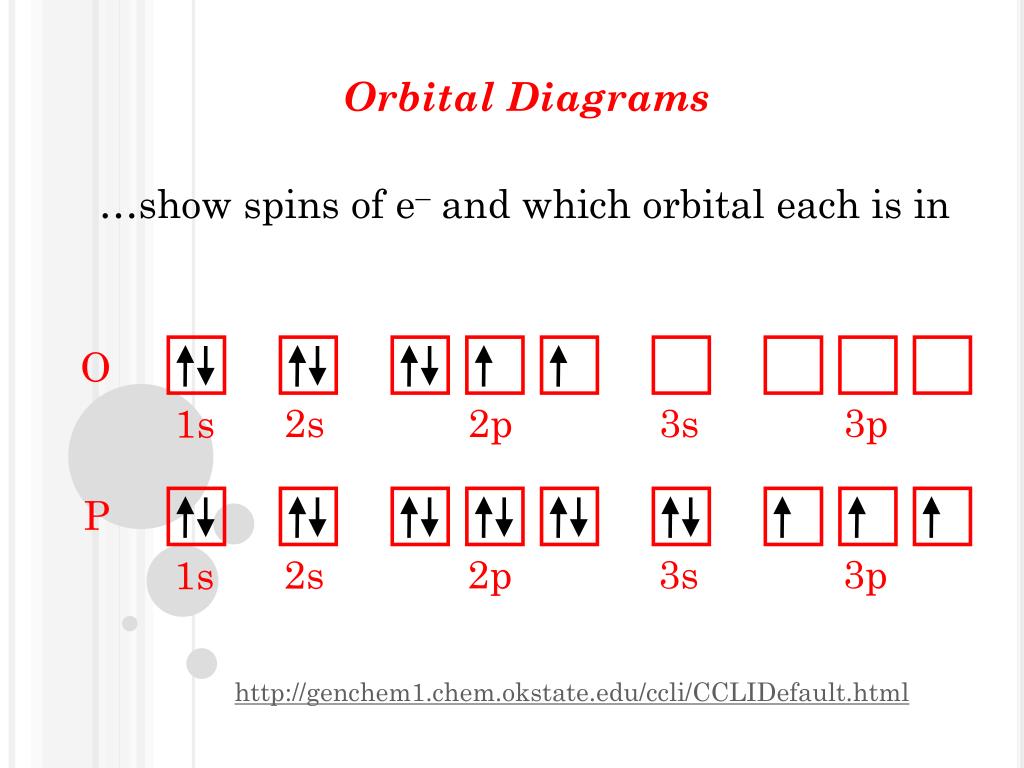
Orbital diagram for carbon. Explanation of the missing 1-s orbital electrons of carbon ... Explanation of the missing 1-s orbital electrons of carbon in the molecular orbital diagram of methane. Ask Question Asked 1 month ago. Modified 1 month ago. Viewed 99 times 1 $\begingroup$ Consider the molecular orbital diagram of methane, for example found here: I would like to know what happens with the two 1s orbital electrons of carbon in ... Carbon Monoxide Molecular Orbital Diagram Explanation There are 4 electrons in the outer shell of carbon and 6.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Co2+ Orbital Diagram - schematron.org This is because we use a π orbital twice, which isn't possible. The second diagram corrects this by realizing there are two unused p orbitals on the carbon. The valence electron configuration of "O" is ["He"] 2s^2 2p^4. To accommodate the two lone pairs and the bonding pair, it will also form three equivalent sp^2 hybrid orbitals. Electron Configurations and Orbital Box Diagrams ... The electron configuration for carbon is 1s 2 2s 2 2p 2. An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down.
Carbon Bohr Model - How to draw Bohr diagram for Carbon(C ... Here, we will draw the Bohr diagram of the Carbon atom with some simple steps. Steps to draw the Bohr Model of Carbon atom 1. Find the number of protons, electrons, and neutrons in the Carbon atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Which is the correct orbital diagram for carbon? 2s 2p OB ... In a neutral carbon atom, the 1s sublevel has one orbital with two electrons with opposite spins, represented by the arrows pointing in opposite directions. The 2s sublevel also has one orbital with two electrons, also with opposite spins. The 2p sublevel has three orbitals. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... How do you write the orbital diagram for carbon class 11 ... Orbital diagram is the filling of the electrons into different orbitals according to the number of electrons present in an atom and an orbital consists of a maximum of two electrons. Keep in mind that the electrons in an orbital are first singly filled before pairing occurs. Now you can easily draw the orbital diagram for a carbon atom.
Carbon Orbital diagram, Electron configuration, and ... The orbital diagram for Carbon is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Carbon orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest two electrons in the 2p orbital. An orbital diagram for a ground-state electron configuration of Carbon atom is shown below- Which is the correct orbital diagram for carbon? - Brainly.com Carbon is a p-block element. It is the 6th element on the periodic table and therefore it has 6 electrons. The sub-level notation is given as: 1s² 2s² 2p² The s-sublevel can only accommodate two maximum electrons because it has one orbital. This is why both 1s and 2s contains just two electrons each. Orbital Diagram Of Carbon Before Sp3 Hybridization Carbon is making 2 s and 2 p bonds to the oxygen atoms. The 2 s bonds indicate that there are 2 equivalent molecular orbitals formed. To form 2 hybrid molecular orbitals, we need to mix 2 atomic orbitals, an s orbital and a p orbital. The resulting hybrid orbitals are called sp hybrids. sp 3 Hybridization. CO Lewis Structure, Geometry, and Hybridization ... The hybridization of carbon monoxide is sp as its geometrical structure is linear. The below mention diagram is the valence shell electronic configuration of both the carbon and oxygen atom. The half-filled sp(z) hybrid orbital of the carbon atom head-on overlaps with the half-filled sp(z) hybrid orbital of the oxygen atom.

Pmages: carbon bohr models | Carbon Atom Bohr Model Proton Neutron Electron Illustration — Stock ...
Electron configuration for Carbon (element 6). Orbital diagram Carbon electron configuration. ← Electronic configurations of elements. Electronic configuration of the Carbon atom: 1s 2 2s 2 2p 2. Reduced electronic configuration C: [He] 2s 2 2p 2. Below is the electronic diagram of the Carbon atom Distribution of electrons over energy levels in the C atom. 1-st level (K): 2.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
How to Write the Orbital Diagram for Carbon (C) - YouTube To write the orbital diagram for the Carbon atom (C) first we need to write the electron configuration for just C. To do that we need to find the number of electrons for the C atom (there are 6...
Solved Write the orbital diagram of carbon before sp ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (6 ratings) Transcribed image text: Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.

Why does the dz2 orbital have a different shape to the other four d orbitals Kindly answer sir ...
Carbon Monoxide Molecular Orbital Diagram Explanation Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. Hydrogen. Jan 7, The electronic configuration of carbon and oxygen atom are 1s²2s²2p² and 1s²2s²2p⁴ respectively. There are 4 electrons in the outer shell of carbon and 6.
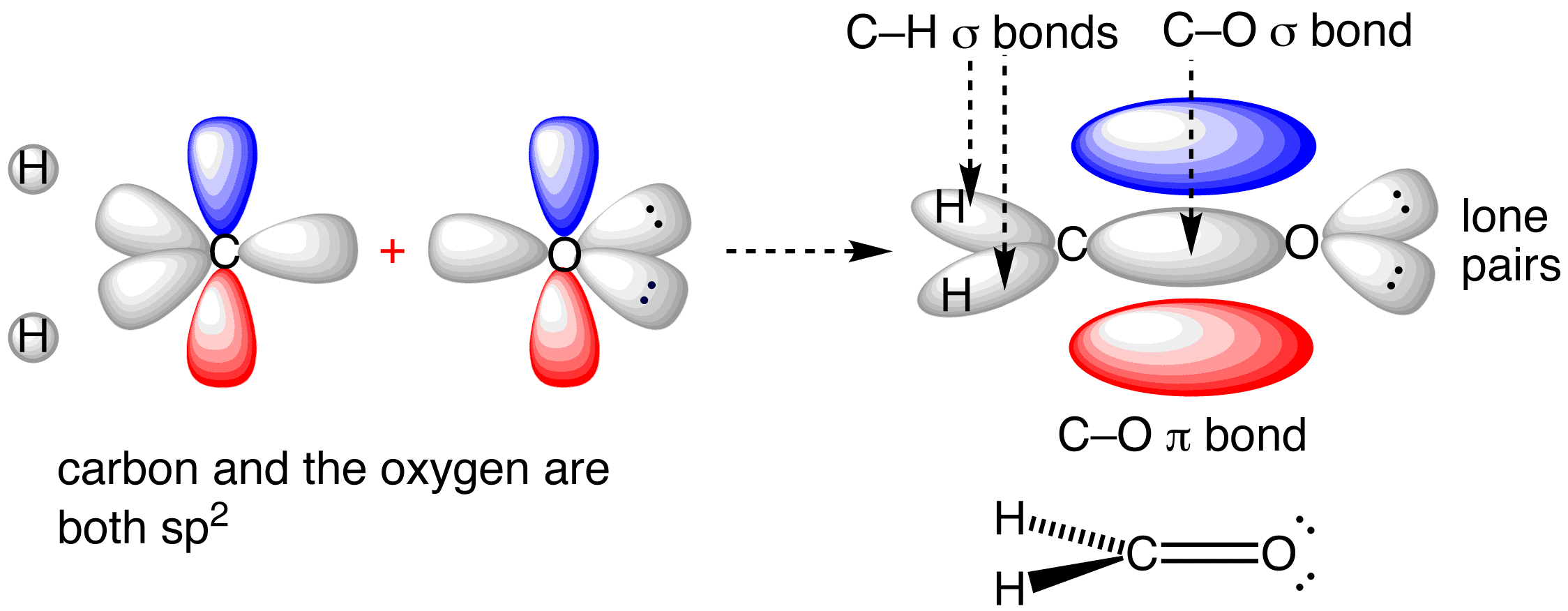
Supplementary Illustrations - Michigan State University Note that the carbon 1s orbital is omitted from the diagram, since it does not contribute to the bonding. Methane Molecular Orbitals. In the following model, the carbon atom is dark gray and the hydrogens are cyan. The hydrogen atoms are arbitrarily numbered. A molecular orbital will be displayed by pressing the appropriate button.The different ...
13.3. Molecular orbitals for three-carbon systems ... Allylic (also called 2-propenyl) carbocations are a common conjugated system. The positive charge of a carbocation is contained in an empty p orbital of a sp2 hybridized carbon. This allows for overlap with double bonds. The positive charge is more stable because it is spread over 2 carbons. Molecular orbitals of an allyl carbocation
Carbon Oxides - University of Illinois Urbana-Champaign Carbon dioxide is electron-poor at the central carbon and acts as an electrophile. The molecular orbital diagram of carbon monoxide is very similar to that of molecular nitrogen. Carbon, with 4 valence electrons, and oxygen with 6 valence electrons, together have the same number of electrons as dinitrogen.


0 Response to "45 orbital diagram for carbon"
Post a Comment