45 boron lewis dot diagram
Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. which lewis electron dot diagram represents a boron atom ... The Lewis-dot structure of boron atom is shown below. Advertisement Advertisement New questions in Chemistry. How many grams of NH3 can be produced from 4.06 mol of N2 and excess H2 . WRITE THE NAME, MOLECULAR AND CONDENSED FORMULA OF ALKYL GROUPS WITH THE FOLLOWING NUMBER OF CARBONS1,2,3,4,5,6,7,8,9,10,11,12
The three dots in the Lewis dot diagram for boron indicate ... The three dots in the Lewis dot diagram for boron indicate that it $\begin{array}{1 1} \text{(a) can bond with three other atoms.} \\ \text{(b)can only form triple covalent bonds.} \\ \text{(c) has three valence electrons.} \\ \text{(d) scores a 3 on Pauling's electronegativity scale.} \end{array} $
Boron lewis dot diagram
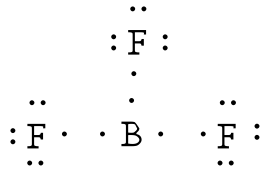
Lewis Dot Diagram - Organic Chemistry - Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE. Lewis Structure of Boron Trifluoride (BF3) Boron trifluoride contains one boron atom and three fluorine atoms. Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of BF 3 with all theories. BF 3 lewis structure BF3 Lewis Structure, Molecular Geometry, and Hybridization BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ...
Boron lewis dot diagram. Boron Lewis Dot Structure | Borates Today Lewis structures, also called Lewis dot formulas or electron dot shapes (LEDs), are diagrams showing the bonding between atoms and the possible lone pairs of electrons within a molecule. Lewis structures can be drawn for any covalently bound molecule and coordination compounds. The Lewis structure is named after Gilbert N. Lewis. How to Draw the Lewis Dot Structure for BN: Boron nitride ... A step-by-step explanation of how to draw the BN Lewis Dot Structure (Boron nitride).For the BN structure use the periodic table to find the total number of ... Lewis Dot Diagram For Boron An electron 13, Electron dot diagram for boron.Lewis dot diagram structures show three formal alternatives for describing bonding in boron monofluoride. BF is unusual in that the dipole moment is inverted with fluorine having a positive charge even though it is the more electronegative element. %tiNitrogen Lewis Dot Structure:Drawing,Several Compounds ... To draw the lewis dot structure of Nitrogen gas, we have to count outermost shell electrons of Nitrogen that is five with electronic configuration: [He] 2s2 2p3. According to the Octet rule each of the Nitrogen atoms must have 8 electrons in outer most shell. This gives Nitrogen atoms stable configuration.
Boron Lewis Dot Structure: Drawing, Several Compounds and ... The Lewis dot structure of Boron trichlortde enhances the idea about sharing electrons. Boron has three valance electrons and three chlorine atoms have (3*7= 21) valance electrons. Therefore, total amount of valance electrons take place in the formation of BCl3 is 24. How to draw BBr3 Lewis Structure? - Science Education and ... It is represented by dots in the BBr3 Lewis diagram. The BBr3 molecule's core boron atom can be represented as follows: Total outermost valence shell electron of boron atom in BBr3= 3 Total outermost valence shell electron of the bromine atom in BBr3= 7 The BC3 molecule has one central boron and three bromine atoms. Lewis Electron Dot Diagrams - Introductory Chemistry, 1st ... Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. Lewis Dot Diagram Beryllium - schematron.org Lewis Dot Diagram Beryllium. This is the berylium chloride and boron chloride Lewis dot structure. Hydrogen, beryllium, and boron have too few electrons to form an octet. Beryllium Bohr Model The number of electrons in each of Beryllium's shells is [2, 2] and its electron configuration is [He] 2s2. The beryllium atom has a radius of.
Boron Trichloride Lewis Dot Diagram - patent ep0758243b1 ... Boron Trichloride Lewis Dot Diagram - 18 images - predicting the single bonded molecular compounds formed by, lewis electron dot structures ck 12 foundation, lewis dot diagram for boron hanenhuusholli, unit 8 9 molecular geometry and polarity, Boron trifluoride (BF3) lewis dot structure, molecular ... Boron trifluoride (BF 3) lewis structure comprises of three B-F bonds, with boron in a central position and all three fluorine as outer atoms in lewis diagram. The lewis dot structure of BF 3 contains a total of 3 bond pairs and 9 lone pairs. The drawing of the BF 3 Lewis structure is very easy and simple. Let's see how to do it. Lewis Dot Structure for Boron Atom (B) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for B (Boron). I show you where Boron is on the periodic table and how to determine how ma... Boron triiodide (BI3) lewis dot structure, molecular ... Hence, put the boron atom at the central position of the lewis diagram and all three iodine atoms outside to it. 3. Connect outer atoms to the central atom with a single bond In this step, join all outer atoms to the central atom with the help of a single bond. In, BI3 molecule, iodine is the outer atom, and boron is the central atom.
Lewis Electron Dot Diagrams - Help A Lewis Structure is a diagram that shows how valence electrons within a compound are distributed among its atoms. Those electrons that are shared by two atoms are referred to as a covalent bond and represented by a dash. Those electons that are located on a single atom are referred to as lone pairs and represented by two dots.
7.2: Lewis Electron Dot Diagrams - Chemistry LibreTexts A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Lewis Electron Dot Diagrams | Introductory Chemistry - 1st ... Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+ The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Lewis Electron- Dot Structure of boron fluoride BF ... Boron monofluoride is an unstable gas but forms stable ligands when combines with transition metals1, like the CO molecule. The experimental BF bond length has been found to be 1.263 Angstrom. The bond order for the BF bond has been calculated to be 1.4.2 Step 1: Connect the atoms with single bonds.
What is Lewis Dot Structure - Lewis Dot Structure | Chem Helps Lewis Dot Structure 101. Lewis Structure or Lewis Dot Structure is a formula developed to concretely express the bond formation between chemical species (atom, molecule, ion).. This formula is to show the electrons in the last layer of the atom with dots around the symbol of the atom.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis structures, also known as electron-dot or electron-dot diagrams, are diagrams showing the bonding between a molecule's atoms and the lone pairs of electrons that may occur in the molecule. What is the Lewis structure of ammonia? Ammonia has the NH3 equation. It is extremely water-soluble because it is a polar material.
How to draw BCl3 Lewis Structure? - Science Education and ... To sketch the BCl3 Lewis structure by following these instructions: Step-1: BCl3 Lewis dot Structure by counting valence electrons on the boron atom. Step-2: Lewis Structure of BCl3 for counting valence electrons around the terminal chlorine atom. Step-3: Lewis dot Structure for BCl3 generated from step-1 and step-2.
Lewis dot structure of boron? - Answers the Lewis structure of B or Boron would have three small dots posing as electrons. These dots can be placed anywhere around the B symbol.
Lewis Dot Diagram For Boron Lewis Dot Diagram For Boron Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for elements. An electron 13, Electron dot diagram for boron. The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;.
BCl3 Lewis Structure, Molecular Geometry, and ... BCl3 Lewis Structure. Let us apply the lewis dot rules and try to draw the structure of boron trichloride. First of all, we need to calculate the total valence electrons of this molecule, B = 3. C l= 7. 3Cl = 7*3=21. So, total= 21+3= 24. Now, boron is less electronegative, which makes it the central atom.
BF3 Lewis Structure, Molecular Geometry, and Hybridization BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ...
Lewis Structure of Boron Trifluoride (BF3) Boron trifluoride contains one boron atom and three fluorine atoms. Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of BF 3 with all theories. BF 3 lewis structure
Lewis Dot Diagram - Organic Chemistry - Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.

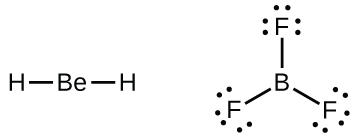




0 Response to "45 boron lewis dot diagram"
Post a Comment