42 molecular orbital diagram he2
› 49212961 › Inorganic_Chemistry_by(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab Patra - Academia.edu AP EAMCET (EAPCET) 2022 Exam - Dates ... - Careers360 Formation of molecular orbitals, linear combination of atomic orbitals (LCAO), conditions for combination of atomic orbitals, energy level diagrams for molecular orbitals, bonding in some homo nuclear diatomic molecules-H2, He2, Li2, B2, C2, N2, and O2
› document › 325957447Solution Manual Brady Chemistry 6TH Edition PDF | PDF | Ion ... For CHCl, the molecular mass of 289 gives a multiple of 6, therefore the formula is C6H6Cl6. 4.23. The formula mass of the empirical unit is 1 N + 2 H = 16.03. Since this is half of the molecular mass, the molecular formula is N2H4. 32.0 g/mol hydrazine x 1 mol NH2/16.03 g = 2 mol NH2/mol hydrazine. 4.24. 3CaCl2(aq) + 2K3PO4(aq) Ca3(PO4)2(s ...
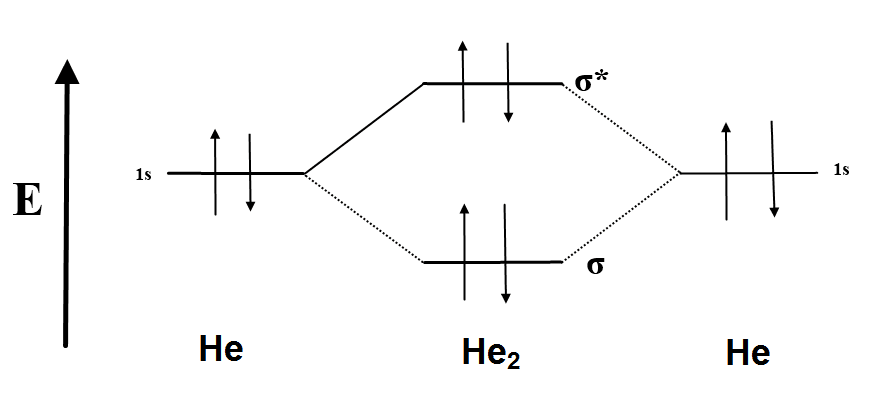
Molecular orbital diagram he2
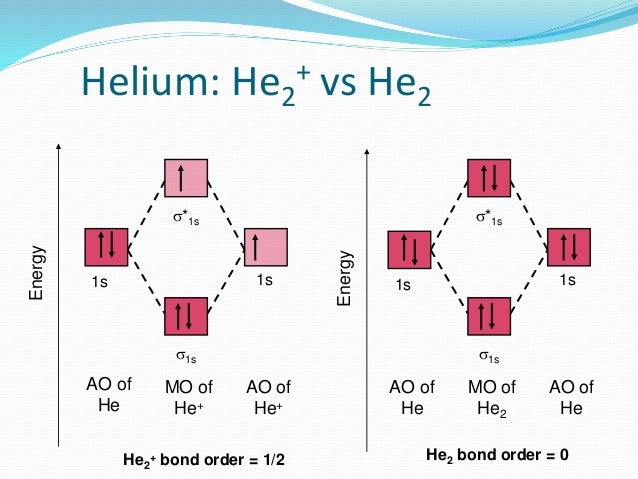
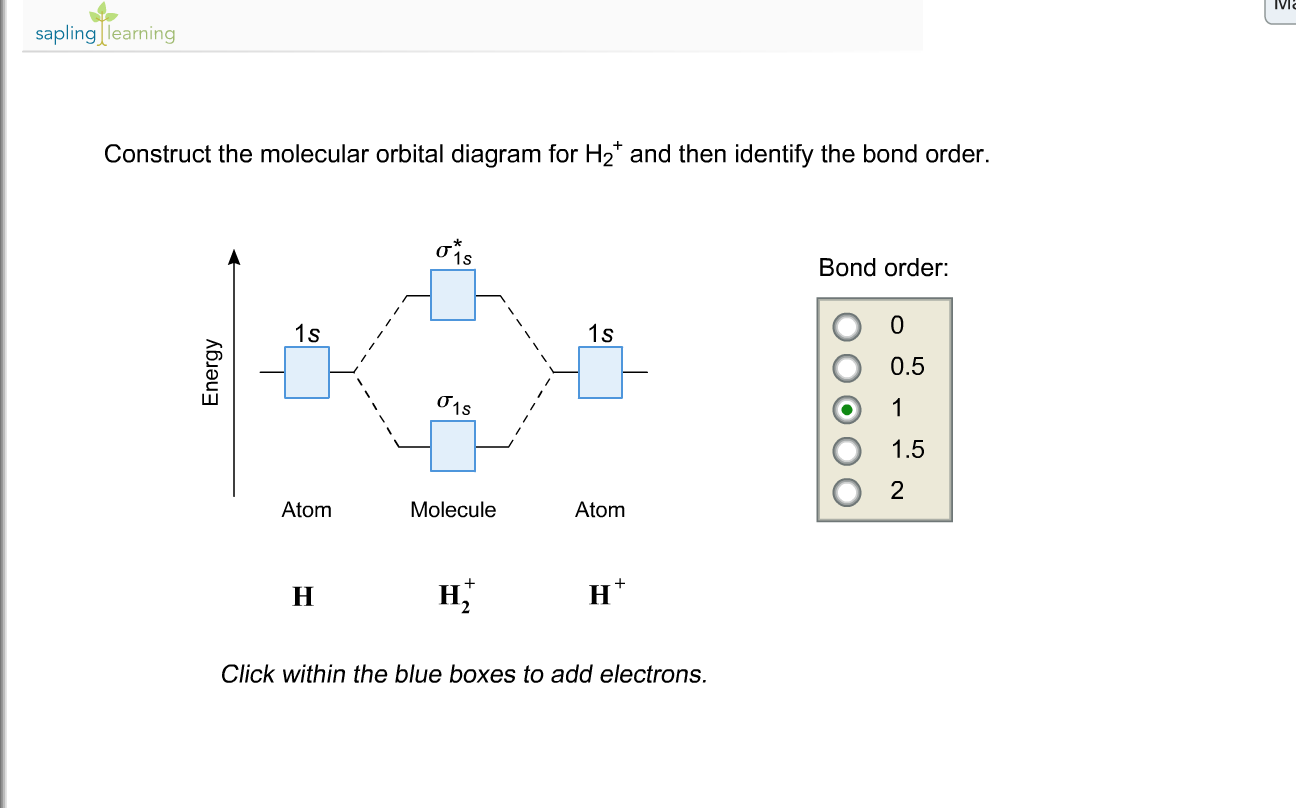
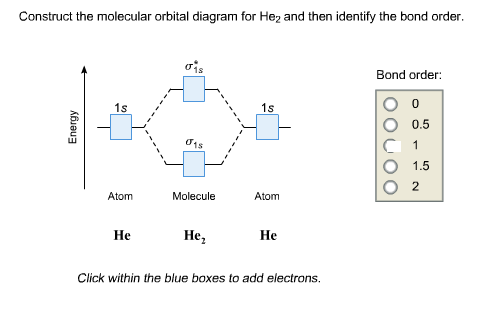
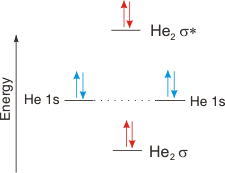
Atoms : Periodic Table, Isotopes, Atomic Structure ... Na + H2O →Na+ (aq) + OH- (aq) + H2 (g) The hydroxides produced are high melting solids which are soluble in water. The great reactivity of these elements is reflected in their atomic structure and their large atomic radius. The atoms lose the outer electron to acquire the electronic configuration of the nearest inert gas. quizlet.com › 538030724 › module-two-chem-101Module Two Chem 101 Problems Flashcards - Quizlet The molecular orbital energy diagram for N₂ is shown below. Based on this diagram, is the molecule paramagnetic or diamagnetic? ... C. He2 Draw the MO diagram for ... courses.lumenlearning.com › introchem › chapterBond Order | Introduction to Chemistry - Lumen Learning The electron configuration of dihelium If the molecule He2 were to exist, the 4s electrons would have to fully occupy both the bonding and antibonding levels, giving a bond order of zero. Dihelium does not exist. Dilithium (Li 2) The last diagram presents the molecule dilithium (Li 2). The 1s electrons do not take part in the bonding, but the ...
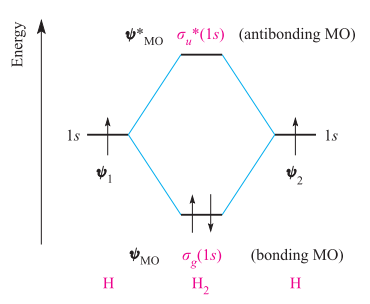
Molecular orbital diagram he2. › 37796622 › Engineering_Chemistry(PDF) Engineering Chemistry by Jain & Jain | Inder Rahi ... Enter the email address you signed up with and we'll email you a reset link. inorganic chemistry - What theory accurately explains ... Since beryllium is an alkaline earth metal, the bonds between beryllium atoms could be considered metallic and we can use molecular orbital theory (MOT) to explain metallic bonds in metals. Consider metallic bonding in lithium metal. Say, n lithium atoms combine to form L i X n. courses.lumenlearning.com › introchem › chapterBonding and Antibonding Molecular Orbitals | Introduction to ... The presence of a filled antibonding orbital, after fulfilling the conditions above, indicates that the bond in this case does not exist. The bonding diagram for the hypothetical molecule He2.Notice the two electrons occupying the antibonding orbital, which explains why the He 2. molecule does not exist. EOF
schematron.org › 2n6-mitosis-diagram2n=6 Mitosis Diagram - schematron.org Mar 16, 2019 · Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order. Astak Cm 918t Wiring Diagram; Fj Cruiser Serpentine Belt Diagram; Doosan D80s Forklift Wiring Diagram; Kenwood Kdc 2011s Wiring Diagram; Ezgo Txt 36 Volt Shift Lever Wiring Diagram; Suzuki Eiger 400 Wiring Diagram And Parts; Arduino Uno Dm542t Wiring Diagram TS EAMCET 2022 Exam: Dates (Out), Registration (Started ... JNTUH has released the TS EAMCET 2022 application form. Check all details about TS EAMCET 2022 exam such as dates, application form, admit card, syllabus, pattern, result, cut off, counselling, selection process and more here. courses.lumenlearning.com › introchem › chapterBond Order | Introduction to Chemistry - Lumen Learning The electron configuration of dihelium If the molecule He2 were to exist, the 4s electrons would have to fully occupy both the bonding and antibonding levels, giving a bond order of zero. Dihelium does not exist. Dilithium (Li 2) The last diagram presents the molecule dilithium (Li 2). The 1s electrons do not take part in the bonding, but the ... quizlet.com › 538030724 › module-two-chem-101Module Two Chem 101 Problems Flashcards - Quizlet The molecular orbital energy diagram for N₂ is shown below. Based on this diagram, is the molecule paramagnetic or diamagnetic? ... C. He2 Draw the MO diagram for ...
Atoms : Periodic Table, Isotopes, Atomic Structure ... Na + H2O →Na+ (aq) + OH- (aq) + H2 (g) The hydroxides produced are high melting solids which are soluble in water. The great reactivity of these elements is reflected in their atomic structure and their large atomic radius. The atoms lose the outer electron to acquire the electronic configuration of the nearest inert gas.
Which Electrons In This Diagram Contribute To The Stability Of The He2 Ion - Free Diagram For ...





0 Response to "42 molecular orbital diagram he2"
Post a Comment