41 what is a lone pair in a lewis diagram
Lewis Structures - Chemistry LibreTexts If you have run out of electrons you are required to use lone pairs of electrons from a terminal atom to complete the octet on the central atom by forming multiple bond (s). In this case the N is short 2 electrons so we can use a lone pair from the left most O atom to form a double bond and complete the octet on the N atom. Solved 1. What is a lone pair in a lewis diagram? (1pt) a ... Question: 1. What is a lone pair in a lewis diagram? (1pt) a. A bonding pair of electrons b. A shared pair of electrons 2 c. A molecule with only two electrons d. An unshared pair of electrons e. The electrons that are not counted when determining if the octet of an atom has been satisfied This problem has been solved! See the answer 1.
N3- Lewis Structure: Drawings, Hybridization, Shape ... In this article, we will know about n3- lewis structure, resonance, molecular geometry, formal charge, structure angle and hybridization. Only three nitrogen atoms make up the azoide ion (N 3 -).Two N=N bonds are present in the Lewis structure of the N 3 - ion. Outside nitrogen atoms contain two lone pairs, whereas the core nitrogen atom has none.
What is a lone pair in a lewis diagram
Lone Electron Pairs | Introduction to Chemistry This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H 2 O. The Lewis dot structure for ammonia, NH3. The lone pair attached to the central nitrogen creates bond angles that differ from the tetrahedral 109.5 °. SO2 Lewis Structure - Lewis Dot Structure | Chem Helps There is 1 lone pair in Sulfur atom. 1 oxygen atom has 2 lone pairs and other oxygen atom has 3 lone pairs. The answer for "Draw a lewis structure for so2 in which all atoms obey the octet rule" can be found above. Lewis Structure For SO2. For some molecules and ions, a single Lewis structure is not sufficient. Clf2 Lewis Structure With Lone Pairs Clf2-is linear while clf2 is v shape why. Add lone pairs to the lewis structure of the polyhalide ion ClF2. Out of the 4 electron pairs 2 of them will be the bond pairs forming bonds with F in Cl-F and the other 2 will be the lone pairs. Add lone pairs to these Lewis structures of polyhalide ions. F- Cl - F.
What is a lone pair in a lewis diagram. NH2OH lewis structure, Molecular geometry, and Bond angle Lone pairs are those represented as dots in the lewis diagram that do not take part in the formation of bonds and are also called nonbonding electrons. So, in the NH2OH lewis structure, there are 4 bond pairs means 8 bonding electrons and 3 lone pairs (1 on central atom + 2 on oxygen atom) are present. How to draw Lewis Diagrams - University of Calgary in Alberta How to draw Lewis Diagrams. An outline of how to detemine the "best" Lewis structure for an example, NO 3-is given below: 1. Determine the total number of valence electrons in a molecule 2. Draw a skeleton for the molecule which connects all atoms using only single bonds. In simple molecules, the atom with the most available sites for bondng is ... Lewis Dot Symbols and Lewis Structures | Boundless Chemistry Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. CO2 Lewis Structure - Learnool In the lewis structure of CO 2, there are two double bonds around the carbon atom, with two oxygen atoms attached to it, and on each oxygen atom, there are two lone pairs. Steps Here's how you can draw the CO 2 lewis structure step by step.
What are lone pairs and how are they represented in a ... And because the non-bonding nitrogen lone pair lies fairly close to nitrogen it compresses the ∠H − N −H bond down from 109.5∘ to approx. 105∘ in ammonia. On other hand, the lone pair explains the basicity of the ammonia molecule. Ammonium ion, N H + 4, is a REGULAR tetrahedron. And they are normally represented by a double-dot ... ClO- lewis structure, molecular geometry, hybrid, polarity ... Lone pairs electrons are the electrons in the lewis diagram that don't take parts in chemical bonding which means they don't form any chemical bond. Whereas the bond pair electrons are the electrons in the lewis diagram that form a chemical bond between atoms, they are also called shared pair electrons. SRO Lewis Structure:Drawings,Hybridization,Shape,Charges ... SRO Lewis Structure Lone Pair. In the Lewis structure of Strontium Oxide zero lone pair present on strontium and two lone pair present on oxygen atom. This is due to the fact that Strontium has lost its two electron i.e. one lone pair and converted to Sr +2 which has no lone pair on it. 7.3: Lewis Symbols and Structures - Chemistry LibreTexts The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is usually used to indicate a shared pair of electrons: In the Lewis model, a single shared pair of electrons is a single bond.
Lone pair - Wikipedia Lone pairs (shown as pairs of dots) in the Lewis structure of hydroxide. In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. NO2 Lewis Structure, Molecular Geometry, Hybridization ... Lewis Structure is a diagrammatic representation of any given molecule with the help of the constituent atoms and the position and arrangement of electrons to form bonds and lone pairs. This is a limited theory on chemical bonding nature and electronic structure but provides a simple viewpoint towards the formation of any molecular composition. Lewis structure - Wikipedia Place lone pairs. The 14 remaining electrons should initially be placed as 7 lone pairs. Each oxygen may take a maximum of 3 lone pairs, giving each oxygen 8 electrons including the bonding pair. The seventh lone pair must be placed on the nitrogen atom. Satisfy the octet rule. Both oxygen atoms currently have 8 electrons assigned to them. Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures.
Lewis Structures: Dot Symbols, Diagrams, Examples, Questions The Lewis Structure also denotes the number of lone pairs of electrons present around the central atom. Lewis Structures can be drawn for ionic, covalent and coordination compounds. However, we can draw it only if we know the molecular formula of the compound. It is also helpful to represent the atomic state of elements.
ClO2- Lewis Structure, Geometry, Hybridization, and ... According to this theory, the presence of two lone pairs of valence electrons on the chlorine atom exerts force and bends the structure giving the bond angle slightly lesser than 109°. We know that a single bond consists of only one sigma bond whereas the double consists of one sigma and one pi bond.
7.3 Lewis Structures and Covalent Compounds - Introduction ... Lewis structure: diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Lewis symbol: symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion. lone pair: two (a pair of) valence electrons that are not used to form a covalent bond.
Lewis Electron Dot Structures: Steps, Examples and Limitations Lewis electron dot structures are the diagrammatic representation of covalently bonded atoms in a molecule. They also show lone pairs of electrons that may exist in the molecule. They are used in the case of covalently bonded molecules as well as coordination compounds. This system was introduced by Gilbert Lewis in 1916.
Lewis Structure Definition and Example - ThoughtCo A Lewis structure is a diagram that shows the covalent bonds and lone electron pairs in a molecule. Lewis structures are based on the octet rule. While Lewis structures are useful for describing chemical bonding, they are limited in that they do not account for aromaticity, nor do they accurately describe magnetic behavior. Definition
PDF bonding pair lone pair - Westfield State University Bonding pairs and lone pairs: since an orbital can hold two electrons we usually talk about electrons in pairs. A bonding pair is the pair of electrons that are being shared. A lone pair are a pair of electrons that are not being shared. Lewis structures are a way of representing the atoms and electrons which constitute a bond.
How many lone pairs are in the best Lewis structure of ... [Multiple bonds are treated as just a single pair.] In NH3, there are 4 pairs (three bonding and one lone pair), and they move to the corners of a tetrahedron with N in the center. Since the molecule's shape is defined by the nuclei, ignore the lone pair. The hydrogens form an equilateral triangle and the N is above the triangle's center.
Difference Between Bond Pair and Lone Pair | Definition ... What is a Lone Pair Lone pair is a pair of electrons that are not in a bond. The electrons of the lone pair belong to the same atom. Therefore, a lone pair is also called a non-bonding electron pair. Although electrons in the innermost shells are also coupled and do not participate in the bonding, they are not considered as lone pairs.
How to Determine the Number of Lone Pairs - Chemistry Steps A negatively charged carbon atom should immediately tell you about a lone pair of electrons. In this case, since the carbon has only three bonds and a negative charge, it must also have a lone pair. This can also be confirmed by using the formula: FC= V - (N + B) -1 = 4 - (N + 3) N = 2, thus, one lone pair of electrons
Lone Pair - Chemistry Definition - ThoughtCo A lone pair is an electron pair in the outermost shell of an atom that is not shared or bonded to another atom. It is also called a non-bonding pair. One way to identify a lone pair is to draw a Lewis structure. The number of lone pair electrons added to the number of bonding electrons equals the number of valence electrons of an atom.
Clf2 Lewis Structure With Lone Pairs Clf2-is linear while clf2 is v shape why. Add lone pairs to the lewis structure of the polyhalide ion ClF2. Out of the 4 electron pairs 2 of them will be the bond pairs forming bonds with F in Cl-F and the other 2 will be the lone pairs. Add lone pairs to these Lewis structures of polyhalide ions. F- Cl - F.
SO2 Lewis Structure - Lewis Dot Structure | Chem Helps There is 1 lone pair in Sulfur atom. 1 oxygen atom has 2 lone pairs and other oxygen atom has 3 lone pairs. The answer for "Draw a lewis structure for so2 in which all atoms obey the octet rule" can be found above. Lewis Structure For SO2. For some molecules and ions, a single Lewis structure is not sufficient.
Lone Electron Pairs | Introduction to Chemistry This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H 2 O. The Lewis dot structure for ammonia, NH3. The lone pair attached to the central nitrogen creates bond angles that differ from the tetrahedral 109.5 °.
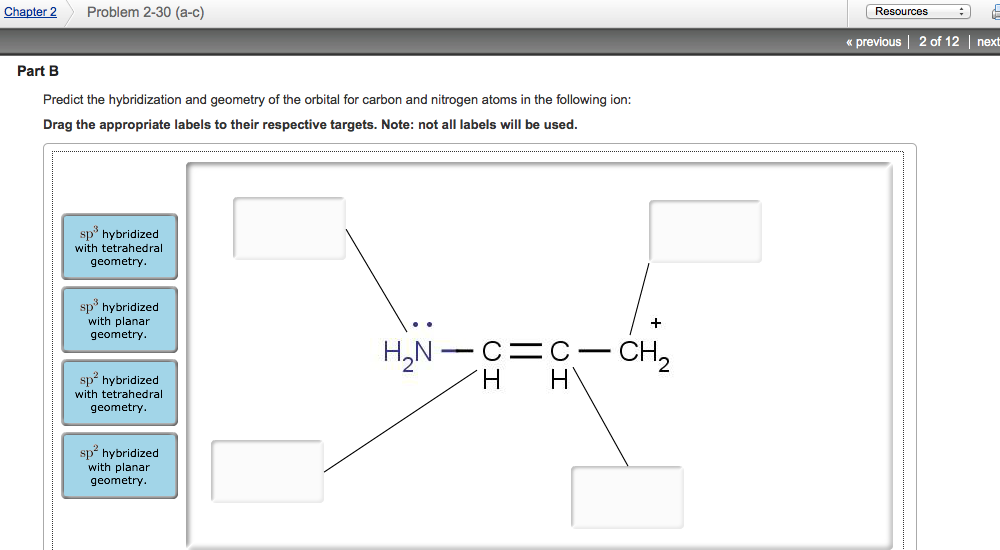


0 Response to "41 what is a lone pair in a lewis diagram"
Post a Comment