41 dry cell battery diagram
Simple Gel cell battery charger circuit - ElecCircuit.com This is the Dry Cell Battery Charger Circuit. This can use a charger battery to get that about 12 hours. When applying to the power supply 9 volts the equipment that fixes in the circuit used for size battery AA. If using the size C or D should devalue of Resistor RX down be 68ohm and should not lead battery comes to serial while voltage in ... Dry Battery Diagram Stock Photo (Edit Now) 83923882 Item ID: 83923882 Dry Battery Diagram Formats 2493 × 2382 pixels • 8.3 × 7.9 in • DPI 300 • JPG 1000 × 955 pixels • 3.3 × 3.2 in • DPI 300 • JPG 500 × 478 pixels • 1.7 × 1.6 in • DPI 300 • JPG Contributor u udaix Similar images See all Assets from the same collection See all Similar video clips
Explain the dry cell with diagram? - Toppr Dry Cell : It is a primary cell based on Leclanche cell invented by G. Leclanche in 1868. In a primary cell, the electrode reactions cannot be reversed by an external source of electrical energy. In this cell, the cell reaction takes place only once i.e., this cell is not rechargeable.

Dry cell battery diagram
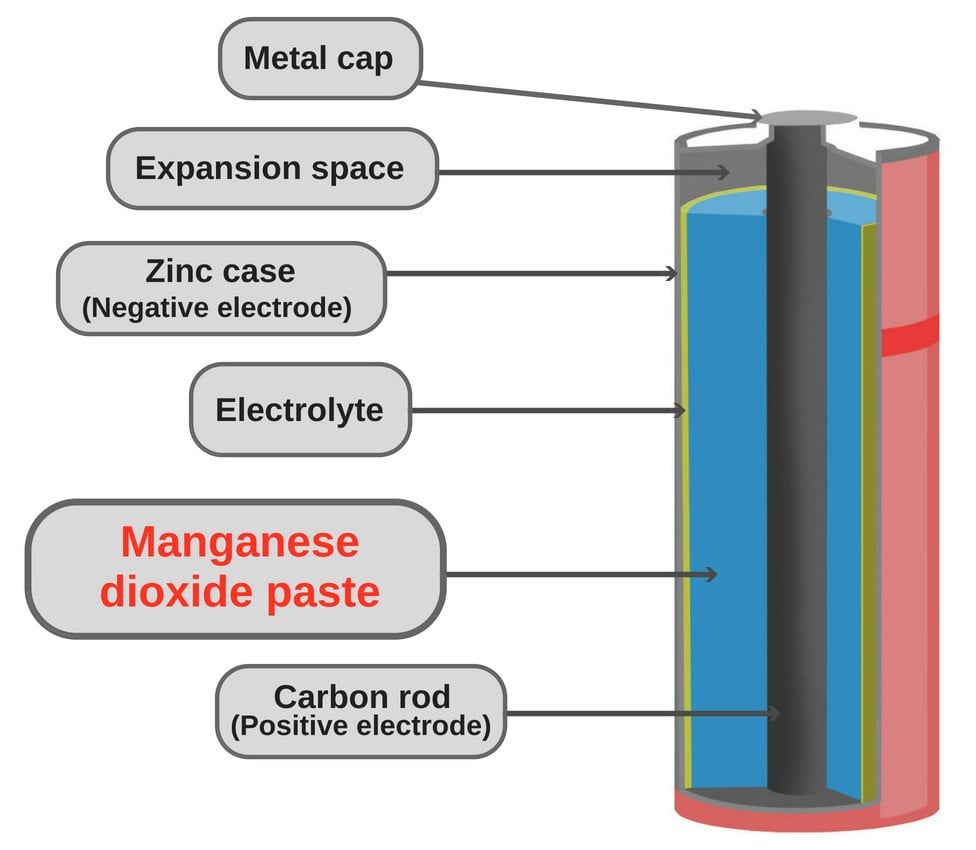
Batteries | Boundless Chemistry - Lumen Learning A common dry-cell battery is the zinc-carbon battery, which uses a cell that is sometimes called the Leclanché cell. The cell is made up of an outer zinc container, which acts as the anode. The cathode is a central carbon rod, surrounded by a mixture of carbon and manganese (IV) dioxide (MnO 2 ). Definition, working principle and types of dry cell - BYJUS A dry cell is one of the electrochemical cells, developed by the "German scientists Carl Gassner" in 1886, after the development of wet zinc-carbon batteries by Georges Leclanche in 1866. Modern dry cells were developed by Yai Sakizo, who is from Japan, in the year of 1887. Dry cell battery structure diagram including parts ... Dry cell battery structure diagram including parts components electrical circuit anode cathode carbon electrode mixture jacket cup metal cover bottom separator sealant barrier film simple easy for science education Image Editor Save Comp Similar Illustrations See All Pricing Help Me Choose More Options Total: $12.00 USD Download Now
Dry cell battery diagram. 11.5: Batteries - Chemistry LibreTexts As shown in part (c) in Figure 11.5.1, a typical lithium-iodine battery consists of two cells separated by a nickel metal mesh that collects charge from the anode. Because of the high internal resistance caused by the solid electrolyte, only a low current can be drawn. Dry Cell Battery - YouTube Check out us at: Cell BatteryA dry cell consists o... Draw Neat and Labelled Diagram of Dry Cell. - Shaalaa.com Draw Neat and Labelled Diagram of Dry Cell. Maharashtra State Board HSC Science (General) 12th Board Exam. Question Papers 255. Textbook Solutions 14289. MCQ Online Tests 73. Important Solutions 4372. Question Bank Solutions 14402. Concept Notes & Videos 821. Time Tables 25. Syllabus. What is Dry Cell battery (Leclanché cell)? and main parts ... =====Dear friends, support this channel ...
Dry cell - Wikipedia A dry cell is a type of electric battery, commonly used for portable electrical devices. Unlike wet cell batteries, which have a liquid electrolyte, dry cells use an electrolyte in the form of a paste, and are thus less susceptible to leakage. The dry cell was developed in 1886 by the German scientist Carl Gassner, after development of wet zinc ... Difference Between Cell and Battery (with Comparison Chart ... The cell and battery both store the chemical energy and then transforms the stored chemical energy into an electrical energy. One of the major difference between the cell and the battery is that the cell is the single unit, whereas the battery is the group of cells. Some other differences between them are explained below in the comparison chart. No. 6 Dry Cells: Options for Vintage ... - Ken's Clock Clinic This 1.5 volt "Long Life" Dry Cell is an exact replacement for the original No. 6 in terms of A-Hr capacity and run time. Folks have loved it because it is renewable. Like the majority of our other No. 6 units, the Model 1900L is protected against accidental short circuits and battery mis-installations (which happens quite frequently). Dry Cell Battery | Introduction to Chemistry A common dry-cell battery is the zinc-carbon battery, which uses a cell that is sometimes called the Leclanché cell. The cell is made up of an outer zinc container, which acts as the anode. The cathode is a central carbon rod, surrounded by a mixture of carbon and manganese (IV) dioxide (MnO 2 ).
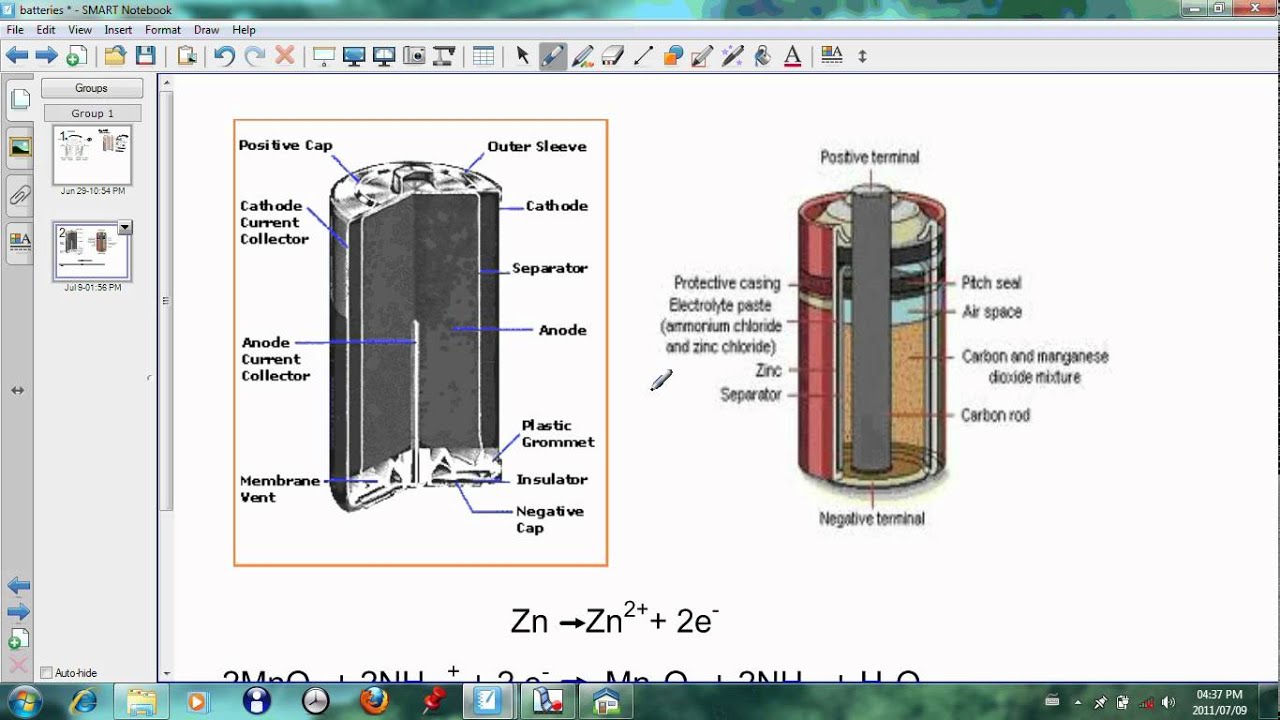
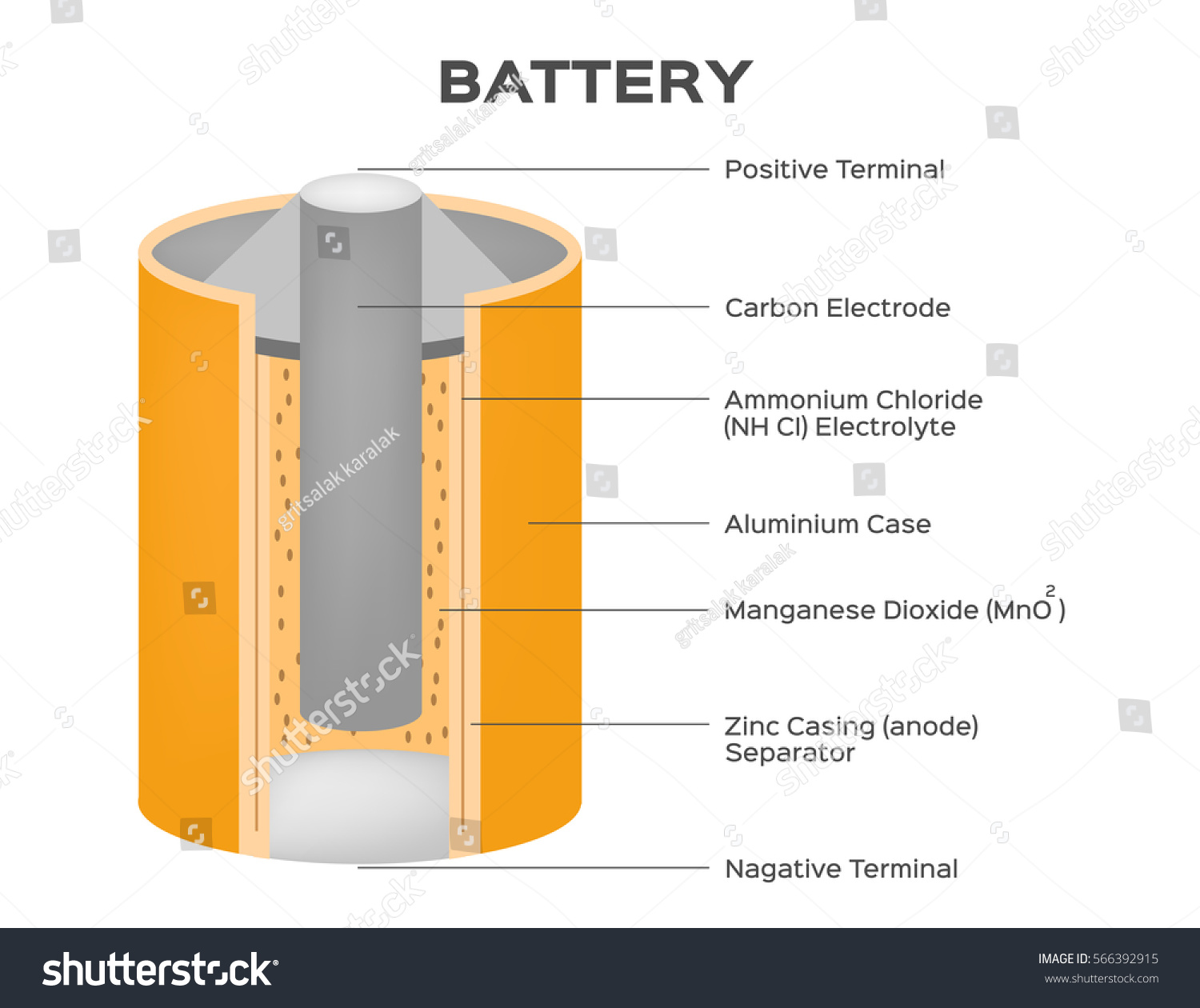
Dry Cell : Structure, Working, Chemical Reactions & Its ... The structure of the zinc-carbon dry cell is shown in the figure. It consists of the anode terminal as zinc or in general graphite rod. The carbon forms the cathode terminal. It may be observed that in older versions of dry cell the zinc was used as cathode and graphite was used as anode terminal. Simple battery structure - Panasonic 1. Electrons generated on zinc plate Electrons are generated on the zinc plate. The zinc atoms which make up the zinc plate leave out some spare electrons, creating zinc irons which break down in the electrolyte solution. The copper plate hardly breaks down at all. 2. Mass transfer of electrons from zinc plate PDF How Batteries Work - My CVEC dry cell, energy is made from a paste of electrolytes. Most of our batteries are dry cells. D ry cells are safer than wet cells. They are also easier to use. 4. A dry cell has 3 parts: the cathode (+), the anode (-), and the electrolyte. The cathode is the tip of the battery with the + sign. The anode is the tip of the battery with the - sign. Electric battery - Wikipedia Line art drawing of a dry cell: 1. brass cap, 2. plastic seal, 3. expansion space, 4. porous cardboard, 5. zinc can, 6. carbon rod, 7. chemical mixture Many types of electrochemical cells have been produced, with varying chemical processes and designs, including galvanic cells, electrolytic cells, fuel cells, flow cells and voltaic piles.
Understanding the Working Principle and Uses of a Dry Cell ... Testing a Dry Cell Battery Things you need: • Multimeter • AA battery 1) Multimeter should be set to read the voltage. 2) The red probe tip should be touched to the top of the battery, whereas that of the black probe should be touched to the bottom of the battery. 3) The multimeter should display the voltage reading of 1.5 volts.
Anatomy of a Battery | HowStuffWorks In normal flashlight batteries, like AA, C or D cell, the terminals are located on the ends. On a 9-volt or car battery, however, the terminals are situated next to each other on the top of the unit. If you connect a wire between the two terminals, the electrons will flow from the negative end to the positive end as fast as they can.
Crosssection Cutaway Diagram Dry Cell Battery Stock Vector ... Find Crosssection Cutaway Diagram Dry Cell Battery stock images in HD and millions of other royalty-free stock photos, illustrations and vectors in the Shutterstock collection. Thousands of new, high-quality pictures added every day.
Amazing Dry Cell Battery Diagram - Best printable template ... Dry cell battery diagram. The modern version was developed by Japanese Yai Sakizo in 1887. The overall reaction is Zn 2MnO 2 2NH 4 Cl Mn 2 O 3 ZnNH 3 2 Cl 2 H 2 O. Diagram of a Dry Cell Battery In the above diagram which represents the parts of a dry cell battery the zinc casing provides enclosing to the electrolyte and the cathode as a whole.
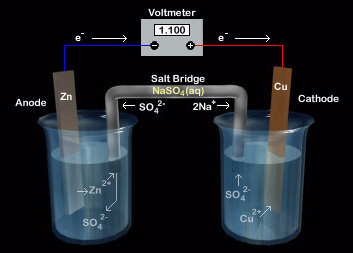
Primary Batteries - Department of Chemistry The reactions that occur in this battery are shown below. Assign the reactions to the correct electrode (cathode or anode). Well done! As in the dry cell, Zn is the anode. Remember: anode = oxidation; cathode = reduction A s in the dry cell, zinc is used as the anode (and container). Look closely at the reduction reaction.
Types Of Battery - Primary cell & Secondary cell - BYJUS An example of a primary battery is the dry cell - the household battery that commonly used to power TV remotes, clocks, and other devices. In such cells, a zinc container acts as the anode and a carbon rod acts as the cathode. A powdered mixture of manganese dioxide and carbon is placed around the cathode.
Leclanche Cell Diagram & Working - Electricalvoice Leclanche Cell Diagram & Working. May 16, 2020. May 14, 2020 by Electricalvoice. The Leclanche cell is a battery which is named after the French scientist Georges Leclanché who invented it in 1866. The Leclanche cell e.m.f. is 1.5 volt. The application of the Leclanche cell was in electric bells, signalling, and telegraphy.
Battery Tester Project - Electronics Project Design Battery Tester Project Using LM3914 IC. This objective of this project is to design and build a battery tester that is able to test various types of dry cell and rechargeable battery with a voltage of less than 2V. Configured as a bar graph battery level indicator, the LM3914 IC from National Semiconductor senses the voltage levels of the ...
battery: zinc-carbon dry cell - Students | Britannica Kids ... The zinc-carbon dry cell (or battery), shown in a cutaway diagram, is a modern version of the Leclanché cell. © Encyclopædia Britannica, Inc.
8.3: Electrochemistry- Cells and Batteries - LibreTexts The dry cell is a zinc-carbon battery. The zinc can serves as both a container and the negative electrode. The positive electrode is a rod made of carbon that is surrounded by a paste of manganese(IV) oxide, zinc chloride, ammonium chloride, carbon powder, and a small amount of water. ... Figure \(\PageIndex{3}\): The diagram shows a cross ...
Dry cell battery structure diagram including parts ... Dry cell battery structure diagram including parts components electrical circuit anode cathode carbon electrode mixture jacket cup metal cover bottom separator sealant barrier film simple easy for science education Image Editor Save Comp Similar Illustrations See All Pricing Help Me Choose More Options Total: $12.00 USD Download Now
Definition, working principle and types of dry cell - BYJUS A dry cell is one of the electrochemical cells, developed by the "German scientists Carl Gassner" in 1886, after the development of wet zinc-carbon batteries by Georges Leclanche in 1866. Modern dry cells were developed by Yai Sakizo, who is from Japan, in the year of 1887.
Batteries | Boundless Chemistry - Lumen Learning A common dry-cell battery is the zinc-carbon battery, which uses a cell that is sometimes called the Leclanché cell. The cell is made up of an outer zinc container, which acts as the anode. The cathode is a central carbon rod, surrounded by a mixture of carbon and manganese (IV) dioxide (MnO 2 ).








0 Response to "41 dry cell battery diagram"
Post a Comment