40 label the phase diagram for carbon dioxide.
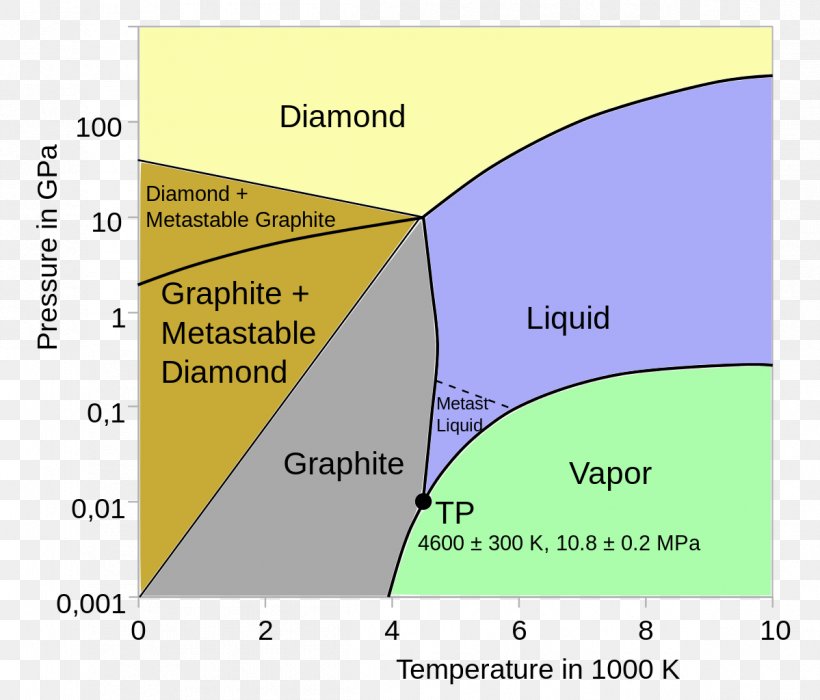
Phase Diagrams · Chemistry Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. PDF Phase Diagram Key - Northern Highlands Regional High School Part C - Phase Diagram for Carbon Dioxide Use carbon dioxide's phase diagram (below right) to answer questions 16-17. 16. At 1 atmosphere and room temperature (25°C), would you expect solid carbon dioxide (at -100°C) to melt to the liquid phase, or sublime to the gas phase? Carbon dioxide will sublime to the gas phase at room temperature. 17.
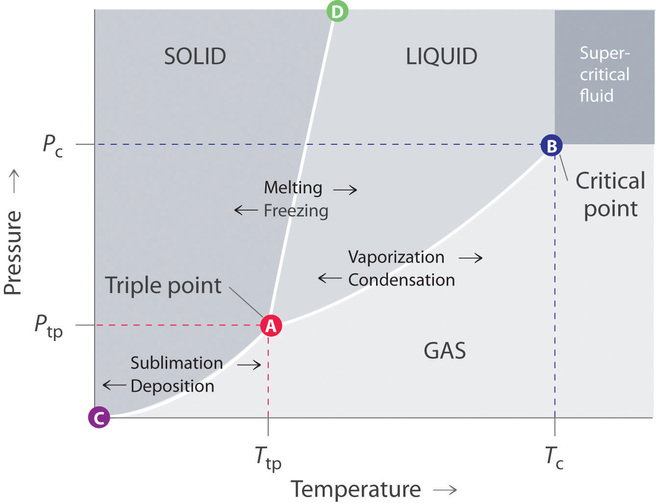
Phase Diagrams - users.highland.edu The phase diagram of carbon dioxide shows that liquid carbon dioxide cannot exist at atmospheric pressure. Consequently, solid carbon dioxide sublimes directly to a gas. Key Takeaway A phase diagram is a graphic summary of the physical state of a substance as a function of temperature and pressure in a closed system.

Label the phase diagram for carbon dioxide.
Phase diagrams (video) | States of matter | Khan Academy It only exists on the moon. And to rebut that comment, I've drawn the phase diagram for carbon dioxide. It's all around you. You're exhaling it as we speak. Your plants in the room are hopefully inhaling it, but carbon dioxide at 1 atmosphere has a very different behavior than water. This is carbon dioxide at 1 atmosphere. umanitoba.ca › outreach › crystalPhase Diagram of Carbon Dioxide - University of Manitoba phase diagrams. for carbon dioxide and water are shown in figure 1. A phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and vapor) of a substance can exist. Both phase diagrams for water and carbon dioxide have the same general . Y-shape, just shifted relative to one another. Phase Diagrams | Chemistry - Lumen Learning Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: On the phase diagram, label the gas and liquid regions. Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
Label the phase diagram for carbon dioxide.. › files › matter_statesChem4Kids.com: Matter: States of Matter Solids, liquids, gases, plasmas, and Bose-Einstein condensates (BEC) are different states of matter that have different physical properties. Solids are often hard, liquids fill containers, and gases surround us in the air. Each of these states is also known as a phase. How does matter change from one state to another? Chem Ch 10 (Exam 1) Flashcards | Quizlet Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (#63) (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. differentiate the phase diagram of water from carbon dioxide From the phase diagram for carbon dioxide in Figure 10.34, determine the state of CO2 at: 80 °C and 10 kPa gas Determine the phase changes that carbon dioxide undergoes as pressure is increased at a constant temperature of (a) -50 °C 2.5 and Fig. Heat content data, heat of vaporization, and entropy values are relative to the liquid state at … PDF Carbon Dioxide: Temperature - Pressure Diagram Carbon Dioxide: Temperature - Pressure Diagram S a t u r at i o n Li ne. Title: phase_diagram.xls Created Date: 11/10/1999 5:44:57 PM
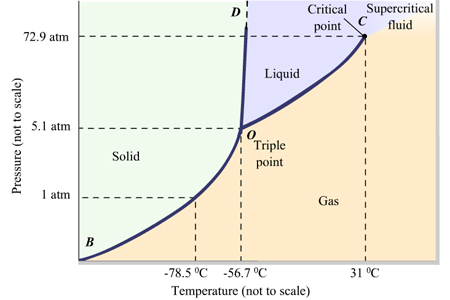
PHASE DIAGRAMS OF PURE SUBSTANCES - chemguide The phase diagram for carbon dioxide The only thing special about this phase diagram is the position of the triple point which is well above atmospheric pressure. It is impossible to get any liquid carbon dioxide at pressures less than 5.11 atmospheres. That means that at 1 atmosphere pressure, carbon dioxide will sublime at a temperature of -78°C. Phase Diagrams | General Chemistry Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). en.wikipedia.org › wiki › Dry_iceDry ice - Wikipedia Dry ice is easily manufactured. First, gases with a high concentration of carbon dioxide are produced. Such gases can be a byproduct of another process, such as producing ammonia from nitrogen and natural gas, oil refinery activities or large-scale fermentation. Second, the carbon dioxide-rich gas is pressurized and refrigerated until it liquefies. Next, the pressure is reduced. When this ... What are the phase diagrams of water and carbon dioxide ... What are the phase diagrams of water and carbon dioxide? Chemistry Phases of Matter Phase Diagrams. 1 Answer Al E. Nov 23, 2017 Each line represents phase changes between respective boundaries. The triple point is where all the phases are at equilibrium. Phase changes are usually isothermal, so temperature is constant until every molecule has ...
OneClass: Label the phase diagram for carbon dioxide. Label the phase diagram for carbon dioxide. Answer +20. Watch. 1. answer. 0. watching. 380. views. For unlimited access to Homework Help, a Homework+ subscription is required. Domarth Duque Lv10. 10 Jan 2021. Unlock all answers. Get 1 free homework help answer. Unlock. Already have an account? Log in ... Phase diagram of carbon dioxide: evidence for a new ... A new phase (CO(2)-II) exists above 20 GPa and 500 K, which can be quenched to ambient temperature. The vibrational spectrum of this new CO(2) polymorph suggests the dimeric pairing of molecules. Based on the present in situ data and previous laser-heating results, we present new constraints for the phase diagram of carbon dioxide to 50 GPa and ... How does the phase diagram for carbon dioxide look like ... For this, complete the following: 1. Roughly sketch the phase diagram, using units of atmosphere and Kelvin. Label the area 1, 2, and 3, and points T and C on the diagram.2. Describe what one would see at pressures and temperatures above 2.0 atm and 450 K. 3. Describe the phase changes from 50 K to 250 K at 1.5 atm. 4. Solved Label the phase diagram for carbon dioxide liquid ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 97% (77 ratings) Transcribed image text: Label the phase diagram for carbon dioxide liquid gas solid-liquid equilibrium liquid-gas equilibrium solid-gas equilibrium triple point solid Temperature ("C ...
pubchem.ncbi.nlm.nih.gov › compound › carbon-dioxideCarbon dioxide | CO2 - PubChem Carbon dioxide is a one-carbon compound with formula CO2 in which the carbon is attached to each oxygen atom by a double bond.A colourless, odourless gas under normal conditions, it is produced during respiration by all animals, fungi and microorganisms that depend directly or indirectly on living or decaying plants for food.
opentextbc.ca › chemistry › chapter10.4 Phase Diagrams – Chemistry On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram. (e) In what phase does carbon exist at 5000 K and 10 8 Pa?
chem.libretexts.org › Phase_DiagramsPhase Diagrams - Chemistry LibreTexts May 03, 2021 · Exception: Water. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to the left as the diagram for water shows.
Phase Diagrams - faculty.chem.queensu.ca Carbon dioxide phase diagram: Look at the features of carbon dioxide's phase diagram. the triple point occurs at a pressure of about 5.11 atm. So we cannot find liquid CO 2 under normal conditions; only solid and vapour.
Solved Label the phase diagram for carbon dioxide. Label ... See the answer Label the phase diagram for carbon dioxide. Label the phase diagram for carbon dioxide. - gas - liquid -triple point soild solid-liquid equilibrium solid-gas equilibrium liquid-gas equilibrium Expert Answer 100% (37 ratings) Previous question Next question
pressbooks-dev.oer.hawaii.edu › phase-diagramsPhase Diagrams – Chemistry On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram. (e) In what phase does carbon exist at 5000 K and 10 8 Pa?
Iron-Carbon Phase Diagram Explained [with Graphs] The weight percentage scale on the X-axis of the iron carbon phase diagram goes from 0% up to 6.67% Carbon. Up to a maximum carbon content of 0.008% weight of Carbon, the metal is simply called iron or pure iron. It exists in the α-ferrite form at room temperature. From 0.008% up to 2.14% carbon content, the iron carbon alloy is called steel.
Phase Diagrams of Water & CO2 Explained - Chemistry ... Phase Changes 2. Freezing - Liquid to Solid 3. Melting - Solid to Liquid 4. Vaporization - Liquid to Gas 5. Condensation - Gas to Liquid 6. Sublimation - Solid to Gas 7. Deposition - Gas to Solid...
10.4 Phase Diagrams - General Chemistry 1 & 2 Using the phase diagram for carbon dioxide shown in Figure 5, determine the state of CO 2 at the following temperatures and pressures: (a) −30 °C and 2000 kPa (b) −60 °C and 1000 kPa (c) −60 °C and 100 kPa (d) 20 °C and 1500 kPa (e) 0 °C and 100 kPa (f) 20 °C and 100 kPa Solution
(Get Answer) - Sketch the phase diagram for carbon dioxide ... Sketch the phase diagram for carbon dioxide. Lable the diagram by dragging the labels to the appr... Sketch the phase diagram for carbon dioxide. Lable the diagram by dragging the labels to the appropriate targets. Note: not all targets will be used. Solution.pdf.
Phase Diagram | Explanation, Definition, Summary & Facts Fig. 4. In order to further explain the phase diagram, we will study the phase diagram of water and carbon dioxide as both compounds have distinct phase diagram and it will give us a better chance to understand different properties of phases of matter.
Photosynthesis Flashcards | Quizlet On diagram 1, fill in the labels with photosynthesis's main inputs of matter and energy. top three, left to right: Sun, Carbohydrates, Oxygen. Bottom two, Left to right: Water, Carbon Dioxide. Complete the following sentence. Photosynthesis is a set of _____ in which_____ energy is converted to _____ energy. ... (Diagram 8) Phase 1, Description ...
Phase Diagrams | Chemistry - Lumen Learning Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: On the phase diagram, label the gas and liquid regions. Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
umanitoba.ca › outreach › crystalPhase Diagram of Carbon Dioxide - University of Manitoba phase diagrams. for carbon dioxide and water are shown in figure 1. A phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and vapor) of a substance can exist. Both phase diagrams for water and carbon dioxide have the same general . Y-shape, just shifted relative to one another.
Phase diagrams (video) | States of matter | Khan Academy It only exists on the moon. And to rebut that comment, I've drawn the phase diagram for carbon dioxide. It's all around you. You're exhaling it as we speak. Your plants in the room are hopefully inhaling it, but carbon dioxide at 1 atmosphere has a very different behavior than water. This is carbon dioxide at 1 atmosphere.






0 Response to "40 label the phase diagram for carbon dioxide."
Post a Comment