40 bf3 molecular orbital diagram
MO_BF3.pptx - Molecular orbital diagram for BF3 1) What is ... molecular orbital diagram for bf3 6) a) determine what irr. representations correspond to the central atom orbitals (look at point group table): b; 2s = a1' (px,py) = e' pz= a2we determined the salcs for the p-orbitals perpendicular to plane of the molecule now perform the same task on the p-orbitals colinear with the bond axis ''5) break down … Is BF3 Polar or Nonpolar?| BF3 Molecular Geometry - What's ... 3. Is BF3 Polar or Nonpolar? BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the Boron-Fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out.
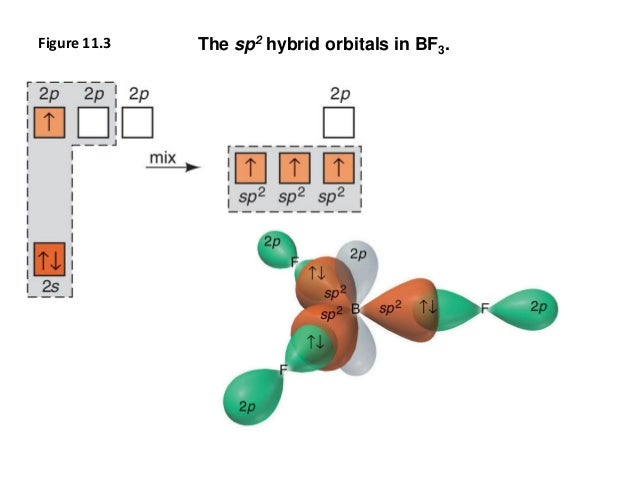
BF3 Lewis Structure, Molecular Geometry, and Hybridization As you found the molecule BF3 is sp2 hybridized (in the presence of 3 orbitals) with 1 boron atom and 3 atoms of fluorine. s-orbital is spherical. 2px and 2py orbitals are in the form of head-to-head loops. All the loops (electrons orbitals) are distanced at a 120-degree angle from each other in the same plane.

Bf3 molecular orbital diagram
PDF MO Scheme of BF Assume that fluorine 2s orbitals are not involved in the bonding and only consider the 2p orbitals. ! The 2p z orbital on each fluorine is perpendicular to the BF 3 plane and capable of forming out-of-plane pi interactions (ð z).! The 2p x orbital points toward the B atom and forms sigma interactions (ó)! The 2p y orbital is parallel to the ... BF3 Lewis Structure (2022 UPDATED) Practical Guide BF3 has fluorine atoms at the top of the equilateral triangle formed with Boron atoms. Thus, its molecular geometry is Trigonal Planar. You will see three atoms that single bond with the central atom. These are three BF bonds at 120o and in the same plane. BF3 Lewis structure, Molecular geometry, Hybridization ... Boron Trifluoride or trifluoroborane (BF3) is an inorganic compound with toxic properties. It is a colorless gas. It has a pungent smell and forms white fumes when exposed to moist air. Boron trifluoride is highly toxic when inhaled. It reacts with heat when exposed for a prolonged period of time and causes a rupture with a blast.
Bf3 molecular orbital diagram. Solved Complete the molecular orbital (MO) diagram of BF3 ... Complete the molecular orbital (MO) diagram of BF3. Assume a ground state electron configuration. Question: Complete the molecular orbital (MO) diagram of BF3. Assume a ground state electron configuration. Diagram Bf3 Orbital Molecular [RH35DQ] Molecular Orbital Theory Bond Order (B. C) When two atoms are connected by a double bond, both of these bonds are π bonds. Second, molecular orbitals, just like atomic orbitals, are arranged in order of increasing energy. Molecular orbital diagram for linear BeH2 5. Bf3 Is Weaker Acid Than Bcl3 And Bbr3. Boron Trifluoride (BF3) - D3h Click the Symmetry Operations above to view them in 3D. BF3 belongs to the D3h point group and contains; one C 3 rotation axis. Three C 2 axes perpendicular to the C 3 Axis. 3σ v planes of symmetry,One σ h plane. An S 3 Axis (not illustrated) Hybridization of BF3 - Hybridization of Boron, Fluoride in BF3 BF 3 molecule is formed by bonding between three sp 2 orbitals of B and p of 3 F atoms. All the bonds in BF 3 are sigma bonds. BF 3 Molecular Geometry and Bond Angles Normally, boron forms monomeric covalent halides which have a planar triangular geometry. This shape is mainly formed by the overlap of the orbitals between the two compounds.
Orbital Molecular Bf3 Diagram [YSWJXE] Search: Bf3 Molecular Orbital Diagram. What is Bf3 Molecular Orbital Diagram. Likes: 601. Shares: 301. Solved 7. Formula: BF3 Lewis Structure Molecular Geometry ... 7. Formula: BF3 Lewis Structure Molecular Geometry: Higongi ponor Orbital Diagram of central atom Before hybridization O Bond Angle: Tao B-f: Is the molecule Polar? non polar Hybridization: sp Orbital Diagram of central atom After hybridization Bonding Scheme of central atom only Bonding Scheme of central atoms and peripheral atoms CIFs Demos 1. Is BF3 Polar Or Nonpolar | All About BF3 | Hybridization ... BF3 Molecular Geometry. The molecular geometry of the BF3 is called trigonal planar. The trigonal planar is a central atom that has three bonded atoms around and has no lone pairs of electrons. That is why the bond and lone pairs' total number becomes three, and the bond form angles of 120°. Hybridization of BF3 -Polarity, Molecular Geometry, Lewis ... BF3 Hybridization. Hybridization as we know is the mixing of atomic orbitals to form new hybrid orbitals. These hybrid orbitals determine the different geometrical shapes of different molecules. Based on the type of orbitals mixed, hybridization is of six types. The type of hybridization seen in a BF3 molecule is sp2.
PF3 and BF3 Molecular Orbitals Correlation of Valence Shell Molecular Orbitals of PF3 and BF3. Click on any line of the table below to view rotatable models of surfaces of these corresponding molecular orbitals for PF 3 and BF 3 respectively; If you scroll back to the line in the table which you clicked, you will see that it is now highlighted as a reminder of which MOs were selected PDF Bf3 lewis structure molecular geometry The BF3 hybridization is to mix atomic orbitals in new hybrid orbitals. They are accommodating to explain the molecular geometry and the properties of nuclear binding. There are different types of hybridization as SP3, SP2, SP. Boron trifluoride | BF3 - PubChem BF3 - Butanol solution, 10 % (w/w) Borane-11B, trifluoro- Fluorure de bore [French] Bortrifluorid trifluoridoboron trifluoro Boron boron trifluride HSDB 325 Trifluoro-borane boron triflouride boron-trifluoride EINECS 231-569-5 UN1008 (11B)Boron trifluoride Boron trifluoride, compressed Leecure B series (Salt/Mix) Boron trifluoride, >=99.5% BF3 Molecular Geometry - Science Education and Tutorials We must now determine the molecular hybridization number of BF3. The formula of BF3 molecular hybridization is as follows: No. Hyb of BF3= N.A (B-F bonds) + L.P (B) No. Hy of BF3= the number of hybridizations of BF3 Number of B-F bonds = N.A (B-F bonds) Lone pair on the central boron atom = L.P (B)

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable ...
BrF3 Lewis Structure, Molecular Geometry, Hybridization ... The above-mentioned diagram shows the MO structure of BF3 (Boron Trifluoride) for your reference. Conclusion In this article, we have discussed the chemical bonding fundamentals of the molecule of BrF3. We have dealt with Lewis Structure, Molecular Geometry, Hybridization, and MO diagram of bromine trifluoride.
BrF3 (Bromine trifluoride) Molecular Geometry, Bond Angles ... 10. Is BF3 Polar or Nonpolar? BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the Boron-Fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out.
Molecular Orbitals for BF3 - Newcastle University Molecular Orbitals for BF3 Jmol models of wavefunctions calculated at the RHF/3-21G* level To view a model, click on a molecular orbital circle in the energy level correlation diagram shown The energy level diagram may be displayed with or without the group theory symbols and character table: the models accessed by clicking are the same.
BF3 Lewis Structure, Molecular Geometry, Hybridization ... How to Draw BF3 Lewis Structure? To draw a Lewis Structure, first of all, add electrons and draw the connectivities. As discussed, here there are 24 electrons. Then, add octets to the outer atom and extra electrons to the central atom. But, as we know, there are no extra electrons. (24 - 24 = 0) Violations
Lecture 20 BF3 Molecular Orbitals Feb 19 2020.pdf - 2/18 ... View Lecture 20 BF3 Molecular Orbitals Feb 19 2020.pdf from CHEM 281 at McGill University. 2/18/20 y Group Theory Approach to Molecular Orbitals x z B, atomic number 5: 1s2 2s2 2p1 F, atomic number
Molecular orbital diagram for BF3 - Chemistry Stack Exchange I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2s for F considered non bonding
Molecular Orbitals of Boron Trifluoride - YouTube The complete molecular orbital scheme of BF3 is developed here.
Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies ...
BF3 Lewis structure, Molecular geometry, Hybridization ... Boron Trifluoride or trifluoroborane (BF3) is an inorganic compound with toxic properties. It is a colorless gas. It has a pungent smell and forms white fumes when exposed to moist air. Boron trifluoride is highly toxic when inhaled. It reacts with heat when exposed for a prolonged period of time and causes a rupture with a blast.

Molecular Orbital Diagram N2 – Bf3 Molecular Orbital Diagram : UNTPIKAPPS - Bf3 Molecular ...
BF3 Lewis Structure (2022 UPDATED) Practical Guide BF3 has fluorine atoms at the top of the equilateral triangle formed with Boron atoms. Thus, its molecular geometry is Trigonal Planar. You will see three atoms that single bond with the central atom. These are three BF bonds at 120o and in the same plane.
PDF MO Scheme of BF Assume that fluorine 2s orbitals are not involved in the bonding and only consider the 2p orbitals. ! The 2p z orbital on each fluorine is perpendicular to the BF 3 plane and capable of forming out-of-plane pi interactions (ð z).! The 2p x orbital points toward the B atom and forms sigma interactions (ó)! The 2p y orbital is parallel to the ...


![[DIAGRAM] Mot Diagram Of Bf3 FULL Version HD Quality Of Bf3 - BPMNDIAGRAMS.GTVE.IT](https://cdn1.byjus.com/wp-content/uploads/2019/07/bf3-molecular-geometry.png)


![[DIAGRAM] 6 Use The D2h Point Group To Construct The Molecular Diagram FULL Version HD Quality ...](https://image1.slideserve.com/2481541/the-mo-diagram-of-methane-n.jpg)

0 Response to "40 bf3 molecular orbital diagram"
Post a Comment