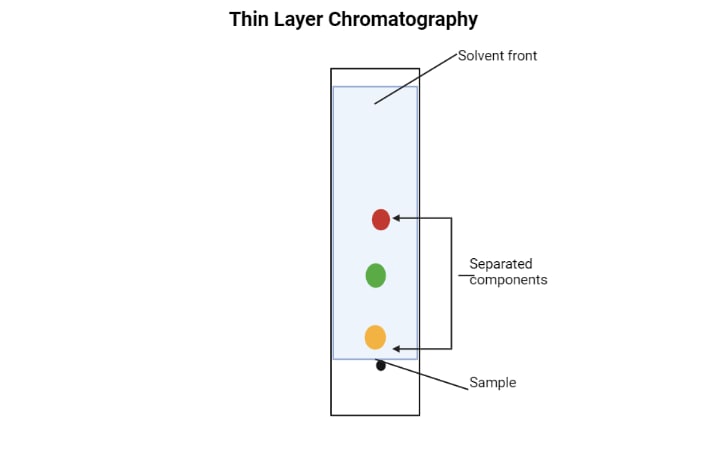
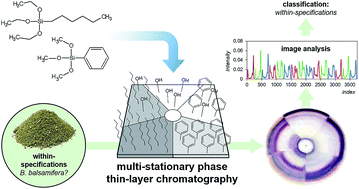
40 thin layer chromatography diagram
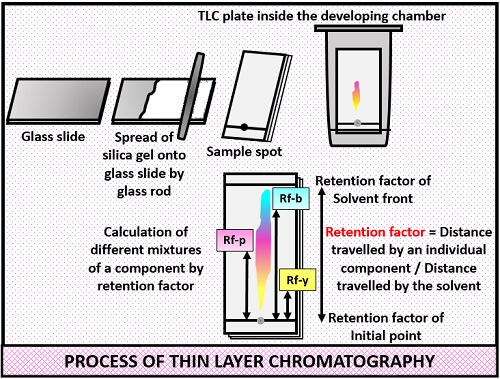
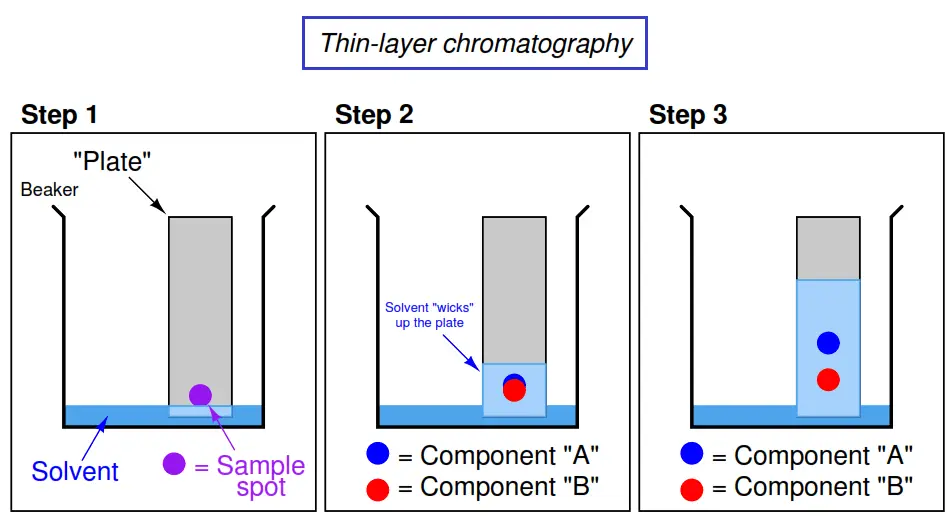
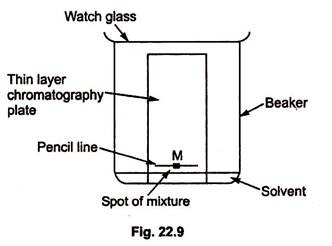
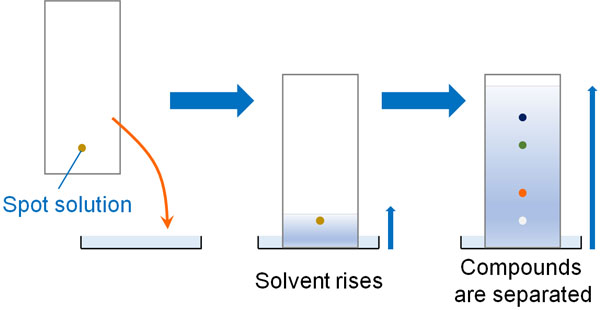
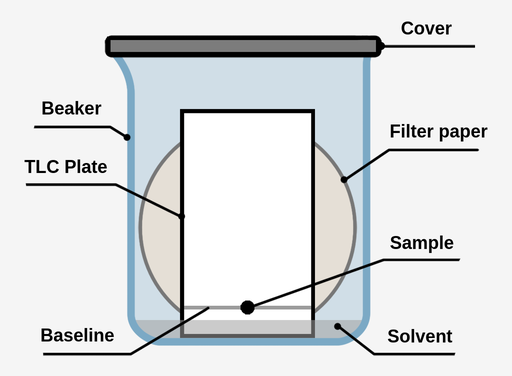
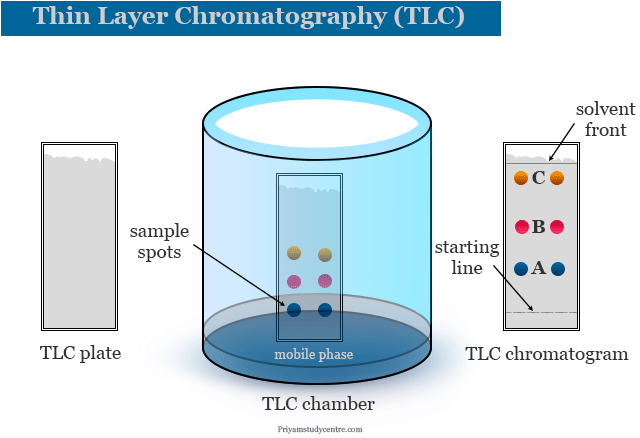
Thin layer chromatography - Chemistry Dictionary Thin layer chromatography works in few steps: Step 1: A horizontal line is drawn near one end (about 1.5 cm from the bottom edge) of TLC plate. In figure below 6 is the horizontal line. Step 2: The sample needs to be separated is placed as a small drop or line on to the TLC plate using capillary tube. Thin Layer Chromatography | Download Scientific Diagram - ResearchGate Download scientific diagram | Thin Layer Chromatography from publication: EVALUATION AND CHARACTERIZATION OF TRANSDERMAL THERAPEUTIC SYSTEMS: AN EXHAUSTIVE PICTORIAL AND FIGURATIVE REVIEW ...
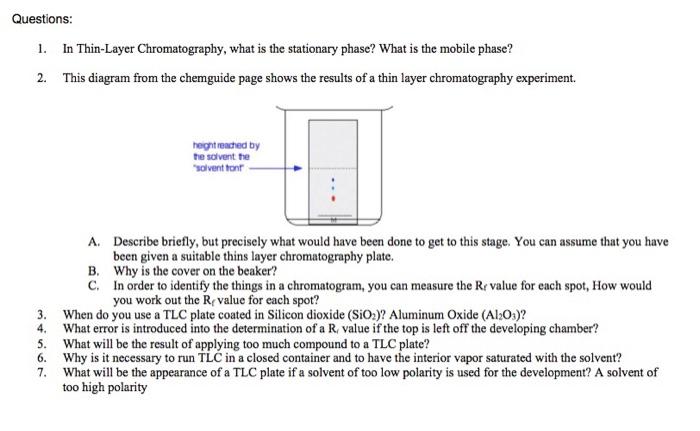
PDF Thin Layer Chromatography (TLC) - Boston College Thin layer chromatography (TLC) is an easy, convenient and inexpensive way to determine how many components are in a mixture and, in many instances, can be used to identify the components as well. In today's experiments, you will gain experience with ... Sketch diagrams of all three chromatograms in your notebook. Measure the
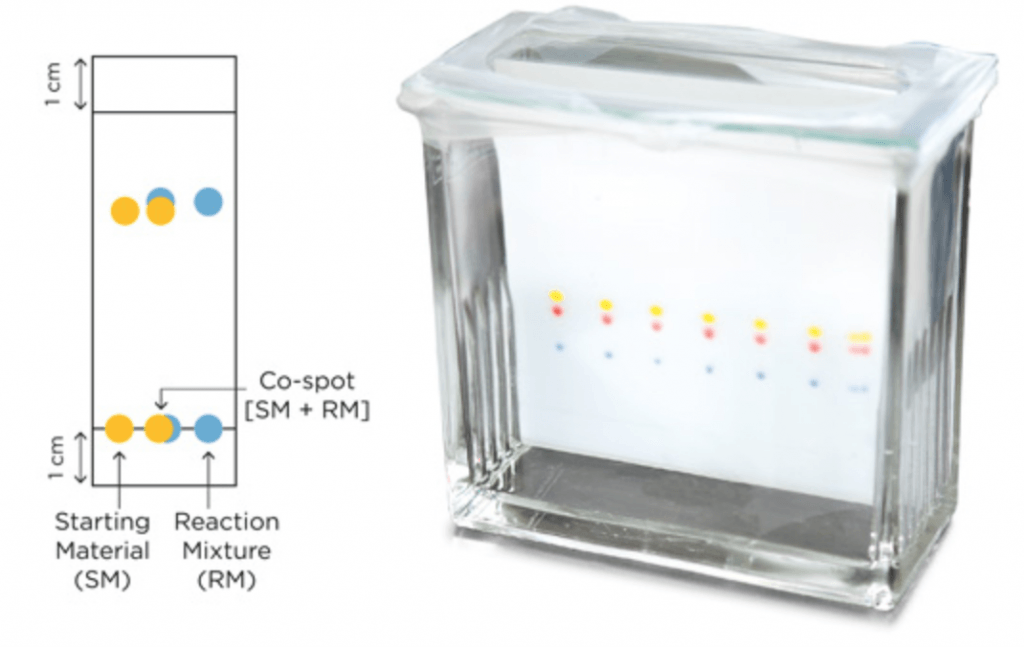
Thin layer chromatography diagram
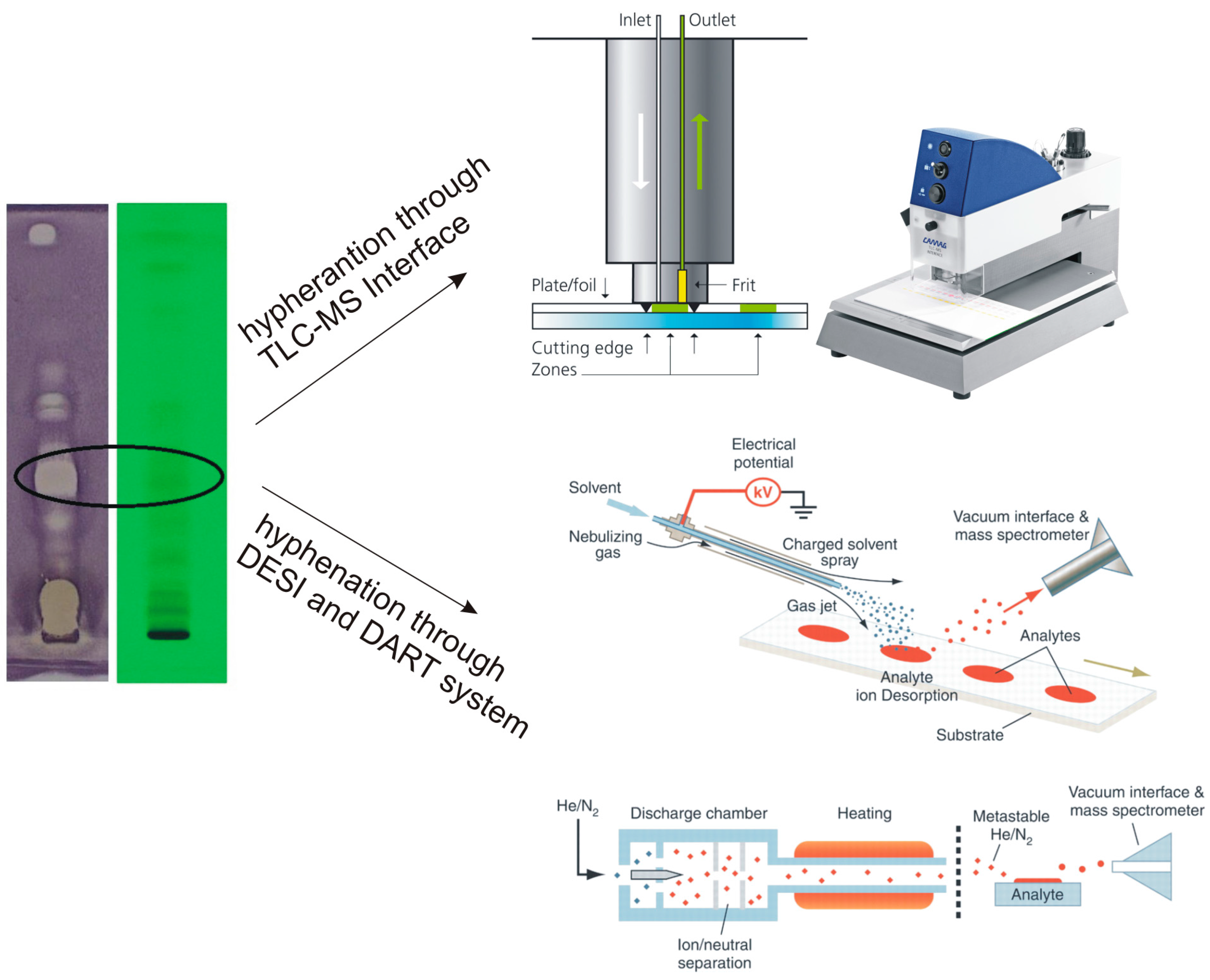
Thin layer chromatography. | Download Scientific Diagram Thin layer chromatography. Source publication Low Pressure, Nonequilibrium-Plasma-Assisted Generation of Flame Retardant Cotton Article Full-text available Jun 2009 Vladimir Totolin Majid Sarmadi... Thin-Layer Chromatography Process The diagram below illustrates the complete thin-layer chromatography process. Sample Preparation Thin-Layer Chromatography plates are economical, single-use products, so sample preparation is simpler or can be avoided completely and is by this more economical than for HPLC. PDF Thin Layer Chromatography - University of Idaho In thin layer chromatography, there is a stationary phase as well as a mobile phase. For this experiment, the TLC plate consists of ... See diagram in introduction. 3. To one plate, spot on the line with any two of the five compounds listed in Table 1. The spots should be about 1 cm apart and on the line.
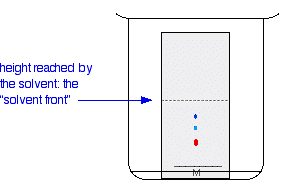
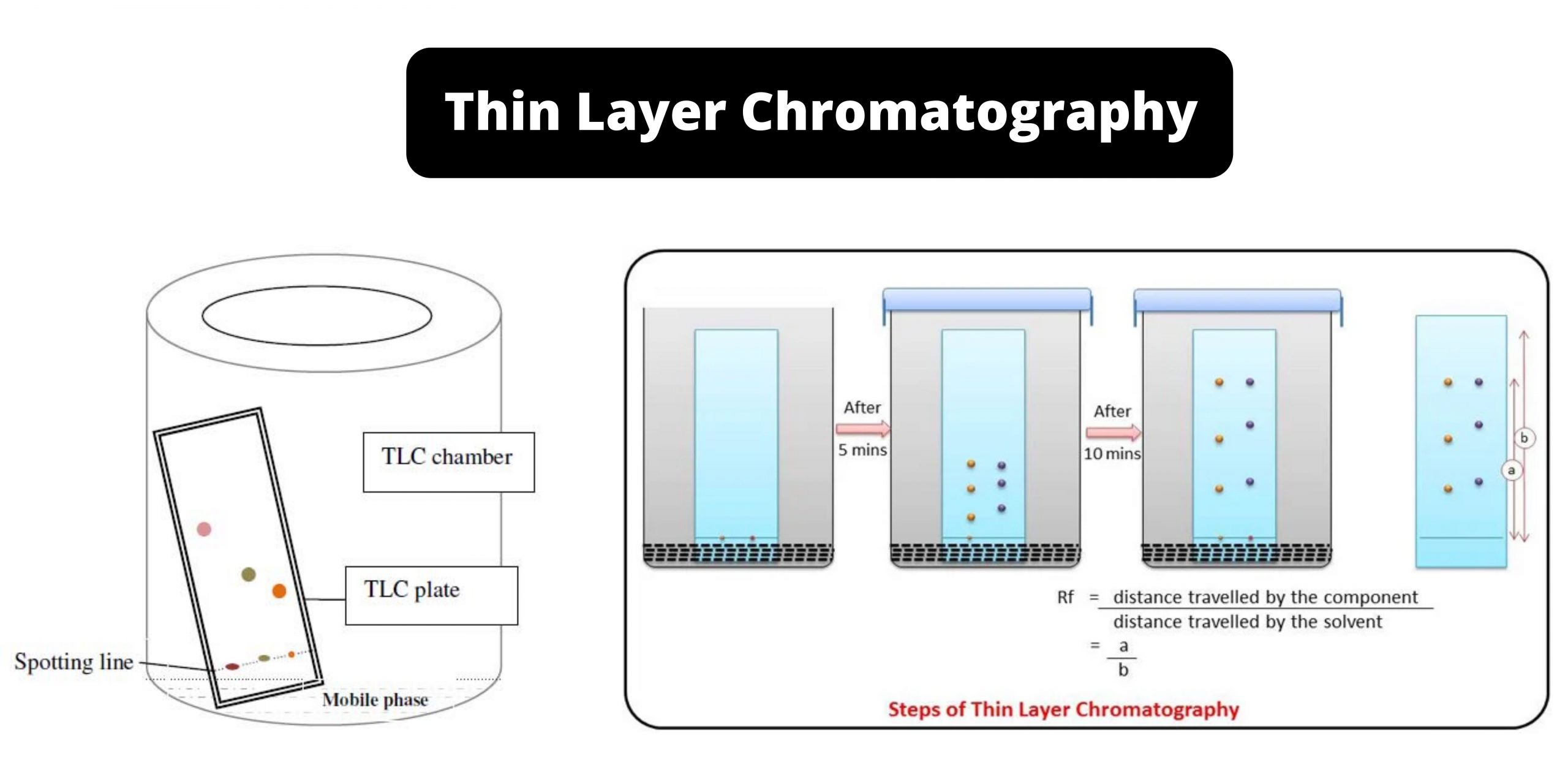
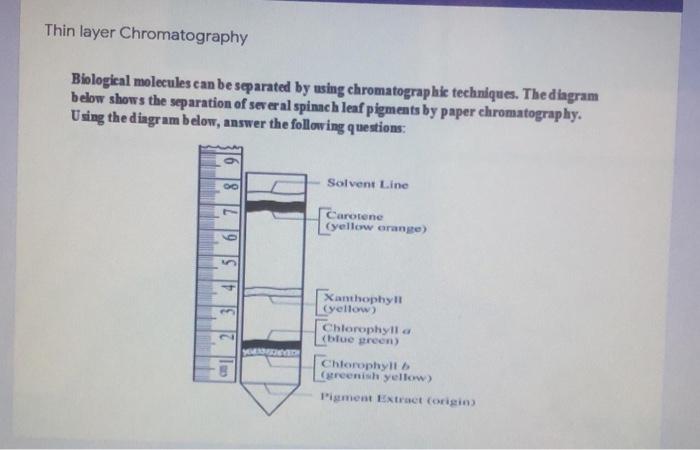
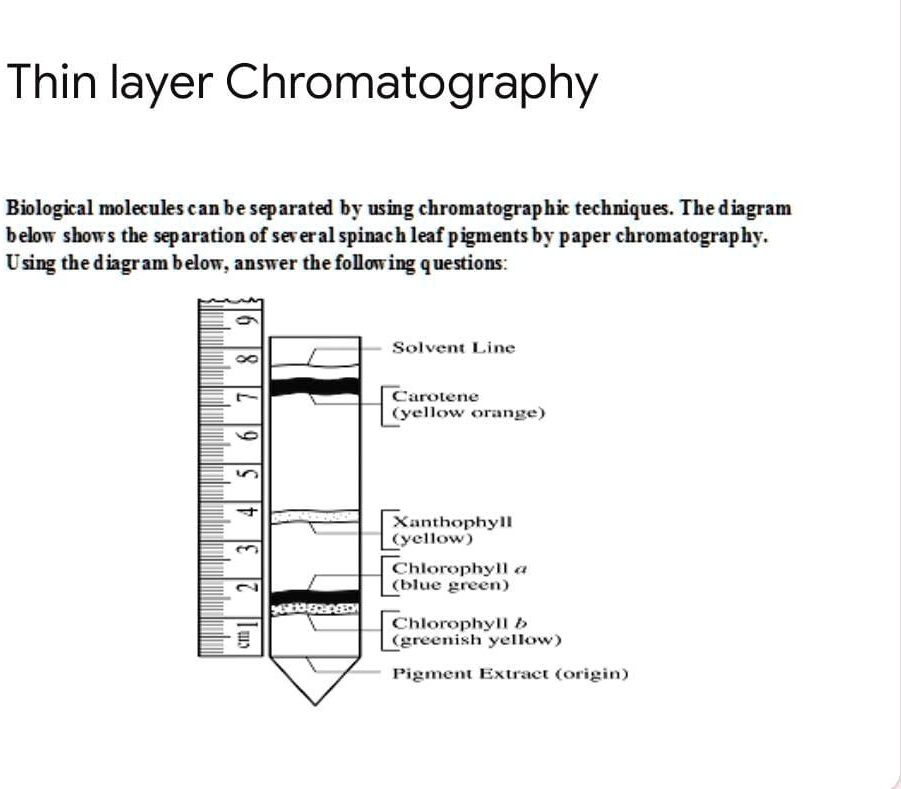
Thin layer chromatography diagram. thin layer chromatography - chemguide A small drop of the mixture is placed on the base line of the thin layer plate, and similar small spots of the known amino acids are placed alongside it. The plate is then stood in a suitable solvent and left to develop as before. In the diagram, the mixture is M, and the known amino acids are labelled 1 to 5. Thin Layer Chromatography - an overview | ScienceDirect Topics Thin-layer chromatography (TLC) as we know it today became established in the 1950s with the introduction of standardized materials and procedures that led to improved separation performance, reproducibility, and a rapid growth in applications. During the 1970s, fine-particle layers and associated instrumentation required for their correct use ... Thin Layer and Column Chromatography - odinity.com Out of all methods to separate compounds, Thin Layer Chromatography (TLC) and Column Chromatography are some of most effective. TLC is typically used for identifying the number of compounds in a mixture as well as their relative polarities as opposed to physically separating them, which can be accomplished with Column Chromatography. ... Thin Layer Chromatography Diagram | Quizlet Thin Layer Chromatography Diagram | Quizlet Thin Layer Chromatography + − Flashcards Learn Test Match Created by Safiyah49 Terms in this set (8) Checking purity of compounds ... Liquid solvent ... A thin layer of SiO2 or Al2O3 on glass ... Solvent front ... Pencil line ... Chromatography plate ... Lid ... Separation by Adsorption
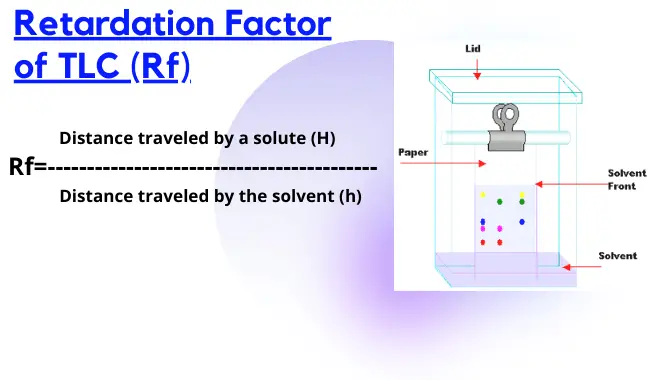
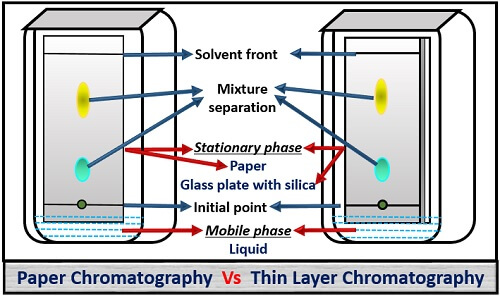
Thin-layer chromatography - Wikipedia Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures. [1] Thin-layer chromatography is performed on a sheet of an inert substrate such as glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide (alumina), or cellulose. Thin Layer Chromatography - Detailed Explanation, and FAQs Working of chromatography is based on the fact that different compounds will have different solubilities and adsorption to the two phases between which they are to be partitioned as shown in the Thin layer chromatography diagram. TLC is a solid-liquid technique in which the two phases are a solid phase (stationary) and a liquid phase (moving). 3 Main Types of Chromatography Techniques (With Diagram) A chromatograph is prepared starting from a single spot. The position of the solved front in marked with pencil as SF1. When the paper is dried up completely, it is rotated by 90° and chromatograph is developed again with a different solvent. The position of the second solvent front is marked SF2. Thin layer chromatography - Chemical analysis - BBC Bitesize Thin layer chromatography (TLC) is similar to paper chromatography but instead of paper, the stationary phase is a thin layer of an inert substance (eg silica) supported on a flat, unreactive...
Thin layer chromatography - PubMed Thin layer chromatography (TLC) is one of the easiest and most versatile methods of doing this because of its low cost, simplicity, quick development time, high sensitivity, and good reproducibility. THIN LAYER CHROMATOGRAPHY - chemguide.uk A small drop of the mixture is placed on the base line of the thin layer plate, and similar small spots of the known amino acids are placed alongside it. The plate is then stood in a suitable solvent and left to develop as before. In the diagram, the mixture is M, and the known amino acids are labelled 1 to 5. Partition Chromatography - Principle, Diagram, Types and ... - VEDANTU Paper Chromatography Diagram - A type of Partition Chromatography ... Thin layer Chromatography - In this type of Liquid -Liquid chromatography, a very thin layer of stationary phase is taken that's why it is known as thin layer chromatography. It is used to separate non-volatile mixtures. Gas ... Thin-Layer Chromatography Practical | StudySmarter So, now let us look at exactly how we can perform a thin-layer chromatography practical. 1. First we need to wear gloves and carefully draw a line 1 cm above the plate using a pencil, then mark 5 equally distanced spots on that line. Gloves prevent any contamination and a pencil is used as it will not dissolve in the solvent.
PDF Thin layer chromatography - University of Victoria Thin layer chromatography (tlc) is an analytical technique for determining the composition of a mixture. It may be used to determine the extent of a reaction, the purity of a compound, or to ascertain the presence or absence of materials in fractions from column chromatography. Tlc analysis may indicate the best conditions for running a column.
Thin Layer Chromatography- Definition, Principle, Parts, Steps, Uses Principle of Thin Layer Chromatography (TLC) Thin-layer chromatography is performed on a sheet of glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide (alumina), or cellulose. This layer of adsorbent is known as the stationary phase.
Thin Layer Chromatography: A Complete Guide to TLC - Chemistry Hall Usually, a thin layer chromatography plate is around 5-7 cm high, and a line is drawn around 0.5-1.0 cm from the bottom. That is the line in which you will spot your mixtures to separate. It is important that you spot the mixtures above the solvent level on your elution chamber!
Thin Layer Chromatography (With Diagram) - Biology Discussion Thin layer chromatography is done exactly as it says-using a thin, uniform layer of silica gel or alumina coated onto a piece of glass, metal or rigid plastic. ADVERTISEMENTS: The silica gel (or the alumina) is the stationary phase. The stationary phase for thin layer chromatography also often contains is substance which fluoresces in UV light.
Thin Layer Chromatography (TLC): Principle, Procedure & Applications Thin layer chromatography is a kind of chromatography used to separate and isolate mixtures that are non-volatile in nature. Just like other chromatography processes, this one consists of a mobile phase and a stationary phase. The latter one here is a thin layer of absorbent material, such as aluminium oxide, silica gel, or cellulose.
Thin Layer Chromatography - Sigma-Aldrich Thin Layer Chromatography. Thin layer chromatography (TLC) is an affinity-based method used to separate compounds in a mixture. TLC is a highly versatile separation method that is widely used for both qualitative and quantitative sample analysis. TLC can be used to analyze virtually any substance class, including pesticides, steroids, alkaloids ...
Thin Layer Chromatography - University of California, Los Angeles Introduction. Thin-layer chromatography (TLC) is a very commonly used technique in synthetic chemistry for identifying compounds, determining their purity and following the progress of a reaction. It also permits the optimization of the solvent system for a given separation problem. In comparison with column chromatography, it only requires ...
Principle and Procedure of Thin Layer Chromatography Thin-layer chromatography is a technique in which the analytes of a mixture are separated by differential migration through the stationary phase. TLC is performed on glass, aluminum or plastic sheets. It is coated with a thin layer of adsorbent material, typically made using silica gel, aluminum oxide, or cellulose and is known as a stationary ...
Thin Layer Chromatography (TLC) - Principle, procedure, Applications on ... Thin Layer Chromatography is a technique used to isolate non-volatile mixtures. The experiment is conducted on a sheet of aluminium foil, plastic, or glass which is coated with a thin layer of adsorbent material. The material usually used is aluminium oxide, cellulose, or silica gel.
Thin layer chromatography Flashcards | Quizlet Draw a base line and any labels at the bottom of the plate with pencil. Put a spot of the each compound or mixture on it. Place in a beaker and add solvent, ensuring that the base line is above the solvent. Cover the beaker and wait. This shows a diagram from the chemguide page, showing a simple thin layer chromatography experiment.
Thin-Layer Chromatography: Definition, Types & Principles - StudySmarter US Thin-layer chromatography, also known as TLC, is a separation technique used to separate and analyse components in a mixture. In chromatography, a mixture dissolves in a solvent, known as the mobile phase, and is carried up a solid, known as the stationary phase.
PDF Thin Layer Chromatography - University of Idaho In thin layer chromatography, there is a stationary phase as well as a mobile phase. For this experiment, the TLC plate consists of ... See diagram in introduction. 3. To one plate, spot on the line with any two of the five compounds listed in Table 1. The spots should be about 1 cm apart and on the line.
Thin-Layer Chromatography Process The diagram below illustrates the complete thin-layer chromatography process. Sample Preparation Thin-Layer Chromatography plates are economical, single-use products, so sample preparation is simpler or can be avoided completely and is by this more economical than for HPLC.
Thin layer chromatography. | Download Scientific Diagram Thin layer chromatography. Source publication Low Pressure, Nonequilibrium-Plasma-Assisted Generation of Flame Retardant Cotton Article Full-text available Jun 2009 Vladimir Totolin Majid Sarmadi...




















0 Response to "40 thin layer chromatography diagram"
Post a Comment