39 lewis dot diagram for selenium
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation Private equity (PE) typically refers to investment funds, generally organized as limited partnerships, that buy and restructure companies.More formally, private equity is a type of equity and one of the asset classes consisting of equity securities and debt in operating companies that are not publicly traded on a stock exchange.
Answer: I'm just guessing; but I'm almost sure you meant SeS2 that is selenium disulfide, not SSe2 that is sulfur diselenide. That is because "S" is a little more electronegative than "Se" and tends to work with a "-2" -non real- oxidation state, but it is true that are almost equal in electrone...
Lewis dot diagram for selenium
What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4. Lewis Diagram For Seo3 A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) . February 23, 2012 - ABOUT · Our Mission · Meet the Team · Partners · Press · Careers · Security · Status · Success Stories · Overview
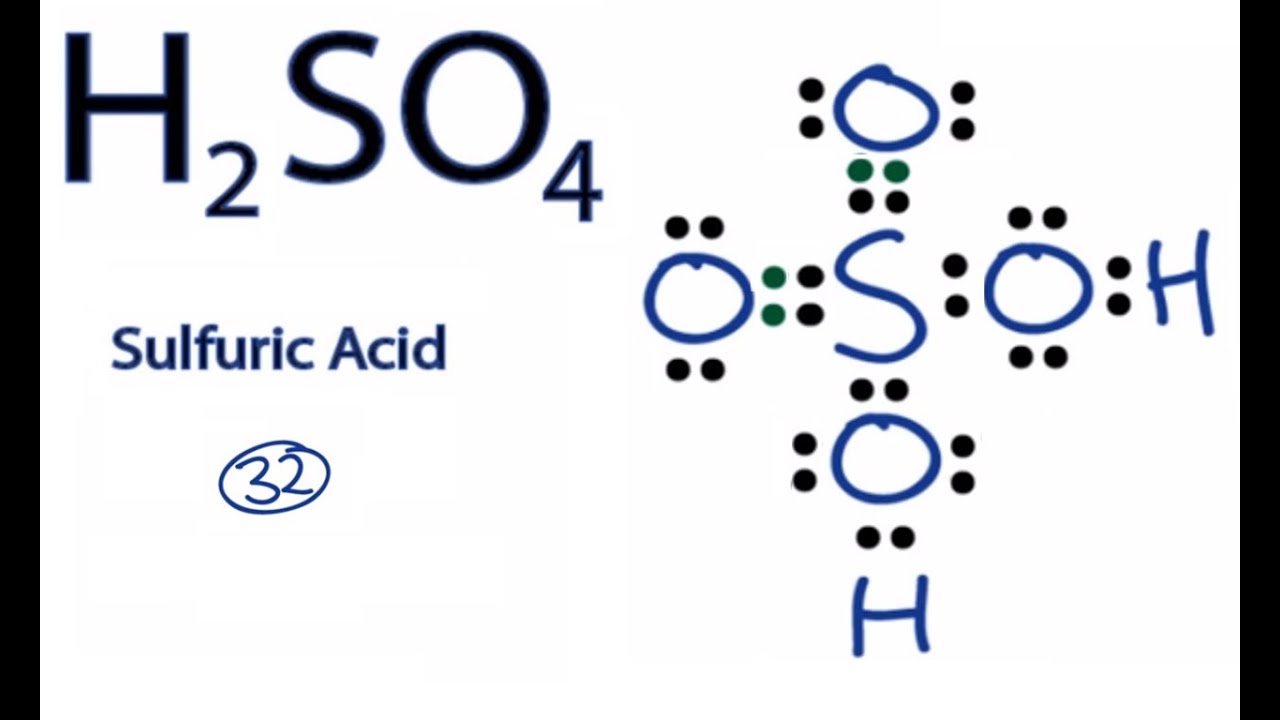
Lewis dot diagram for selenium. A step-by-step explanation of how to draw the SeBr4 Lewis Dot Structure.For the SeBr4 structure use the periodic table to find the total number of valence el... A step-by-step explanation of how to draw the SeF2 Lewis Dot Structure.For the SeF2 structure use the periodic table to find the total number of valence elec... Take A Sneak Peak At The Movies Coming Out This Week (8/12) Why Your New Year’s Resolution Should Be To Go To The Movies More; Minneapolis-St. Paul Movie Theaters: A Complete Guide Draw a Lewis structure for sulfate, SO42-, in which all atoms satisfy, but do not exceed, the octet rule. Now draw a second structure that is the best structure based on formal charge considerations. In the Lewis structures that you’ve drawn, how many single and double bonds surround the central atom in each structure?
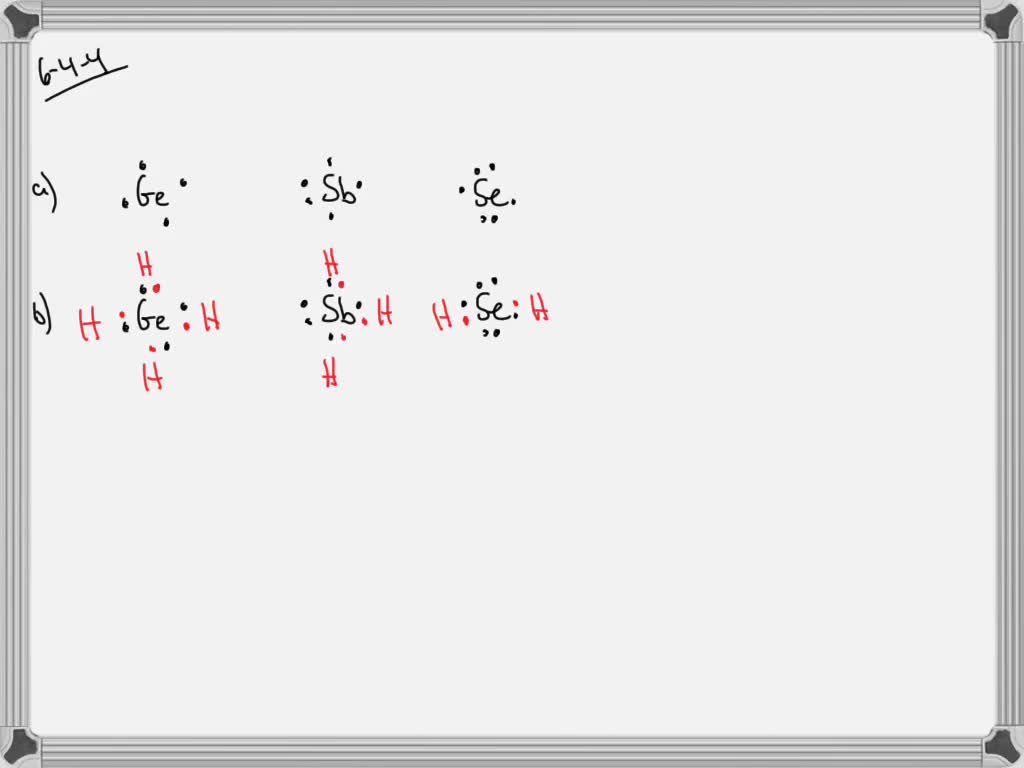
September 18, 2018 - Because selenium is in the 16th column on the periodic chart, it has 6 valence electrons. Thus, the Lewis structure (or electron dot structure) for selenium has 6 dots around it. It will look similar to this without the underscores: __. . Se: _. . Get access to every article of chemistry with in-depth content and well-illustrated images which will help you understand all of the topics of chemistry for board exam as well as competitive exam preparation. Example \(\PageIndex{1}\): What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: \[\dot{Al:} \nonumber\nonumber \] Lewis Structure of Selenium Dioxide (SeO2) Also called the Lewis dot structure, it is a pictorial representation of the behavior and arrangement of valence electrons within an atom. It uses dots to represent valence electrons and lines to show bonds.
Comprehensive data on the chemical element Selenium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Selenium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions ... A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os connected by single lines are both surrounded by six dots, while the other O has four dots. In the Lewis dot structure, the Se represents the element selenium, and each O represents oxygen. Here are a number of highest rated Selenium Lewis Dot Structure pictures on internet. We identified it from reliable source. Its submitted by dealing out in the best field. We recognize this kind of Selenium Lewis Dot Structure graphic could possibly be the most trending topic in the same way as we portion it in google gain or facebook. I know how to draw a Lewis Dot Diagram. Atomic Structure Worksheet. Fill in the blanks for the elements in this chart. Element Symbol. Element. Number of Protons. Number of Neutrons. Number of Electrons. ... Selenium. Magnesium. Helium. Phosphorus. Cesium. Tellurium. Oxygen. Potassium. Beryllium. Carbon.
Drawing the Lewis Structure for SeF 6. Viewing Notes: SeF 6 is a Lewis structure with Selenium (Se) which can hold more than 8 valence electrons. Since there are six Fluorine (F) atoms it will be necessary. The Lewis structure for SeF 6 has 48 valence electrons available to work with.; It's a good idea to check the formal charges for your SeF 6 Lewis structure to make sure they are zero.
Main isotopes of selenium: Se-74, Se-76, Se-77, Se-78, Se-80. Se-79 and Se-82 also exist, but are products of fission. It is a nonmetal. Solid at room temperature. Density- 4.809 g/mL Melting Point- 493.65 K Boiling Point- 958 K Molar Mass- 78.96 g/mol 6 Valence Electrons Electron Configuration- 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p4
H2Se lewis structure contains one selenium atom at the center position and two hydrogens on either side of it. There are a total of 2 lone pairs and 2 bond pairs present in the lewis structure H2Se. The lewis structure of H2Se is very similar to the lewis structure of H2S , this is because both sulfur and selenium have the same valence electron ...
Aug 22, 2021 · The empirical formula for the hydride Selenium would be expected to be _ and its lewis structure would be: Create an account to start this course today Used by over 30 million students worldwide
A step-by-step explanation of how to draw the SeBr2 Lewis Dot Structure (Selenium dibromide).For the SeBr2 structure use the periodic table to find the total...
SeF4 lewis structure is made up of one selenium and four fluorine atoms, selenium is the central atom, and fluorine is kept outside in the lewis diagram. There is one lone pair present on the central atom in the SeF4 lewis structure and 12 lone pairs on outer atoms. Follow some steps for drawing the lewis dot structure of SeF4 1.
It is represented by dots in the H2Se Lewis diagram. The H2Se molecule's core selenium atom can be represented as follows: Total outermost valence shell electron of selenium atom in H2Se= 6 Total outermost valence shell electron of hydrogen atom in H2Se= 1 The H2Se molecule has one central selenium and two hydrogen atoms.
What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4.
In writing an. Answer to orbital diagram for selenium home / study / science / chemistry / chemistry questions and answers / Orbital Diagram For Selenium. here is the electronic configuration. Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4.Selenium (Se) has an atomic mass of Find out about its chemical and physical properties, states, energy ...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
Problem. What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: . The valence electron configuration for selenium is 4s 2 4p 4.In the highest-numbered shell, the n = 4 shell, there are six ...
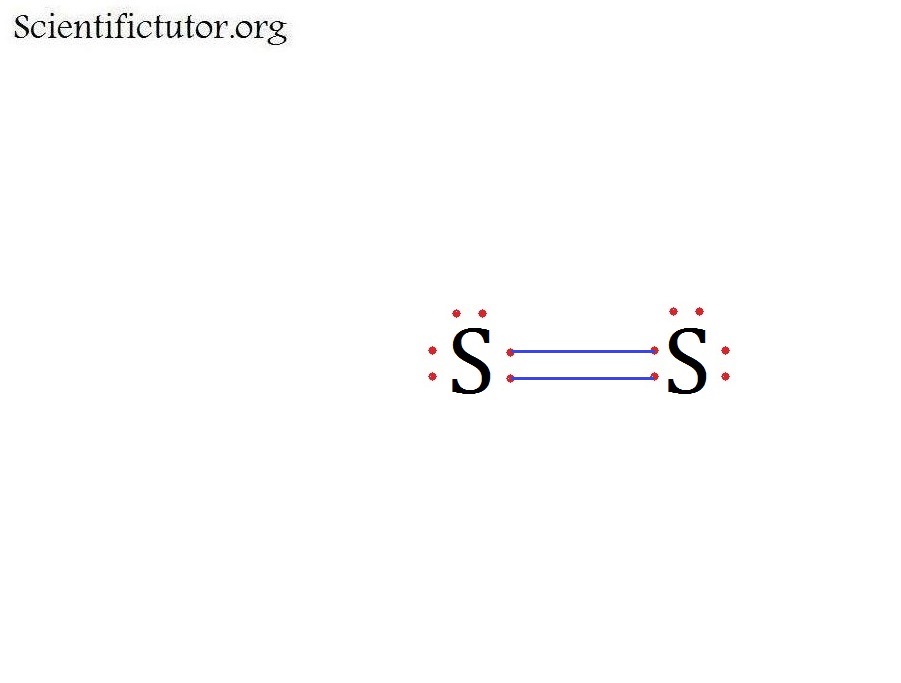
Draw the Lewis dot structure for S2. Selenium sulfide is used as an ingredient in antidandruff shampoos and as a constituent of fungicides. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. How to draw the Lewis Structure of SO2 - with explanationCheck me out. Ne4s 2 4p 6.
Learnfaster.ca is the teaching portal of Pavel Sedach, a private instructor in the Calgary, Alberta Area. As of December 2018, I have moved back to Calgary and am accepting a limited number of students. Tutoring and teaching courses since 2004. An award-nominated teaching assistant (organic ...

U.S. Representative John Lewis poses for a photo at Black Lives Matter plaza in Washington DC (IG: @clay.banks)
A step-by-step explanation of how to draw the SeCl2 Lewis Dot Structure.For the SeCl2 structure use the periodic table to find the total number of valence el...
A Lewis dot structure for an element shows two things: the symbol with an appropriate number of dots representing the proper number of valence electrons. Examples: The standard way of dot placement in a Lewis dot structure has an electron placed in the four directions (North, South, East and West), and then when more than 4 are needed listed ...
A step-by-step explanation of how to draw the Se (Selenium) Se2- Selenide ion) Lewis Dot Structure.For the Se and Se 2- structure use the periodic table to...
Selenium is a naturally occurring mineral element that is distributed widely in nature in most rocks and soils. In its pure form, it exists as metallic gray to black hexagonal crystals, but in nature it is usually combined with sulfide or with silver, copper, lead, and nickel minerals. Most processed selenium is used in the electronics industry, but it is also used: as a nutritional supplement ...
Step 2: Draw the lewis dot structure for elements. We draw the Lewis structure of elements by arranging the valence shell electrons around the element's chemical symbol. The chemical symbols for Selenium and fluorine are Se and F, respectively. The Lewis dot structure for Se and F are as follows-
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.
What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4.
Lewis dot structure is the structure which represent the valence electrons of the element around the chemical symbol of the element. Selenium is the 34th element of the periodic table. Atomic number of bromine is 34 that means it contains 34 electrons, Thus the electronic configuration is represented as: Thus the valence electrons are 6.
February 17, 2021 - Thus, the Lewis structure (or electron dot structure) for selenium has 6 dots around it. ... Lewis structures, also called electron-dot structures or electron-dot diagrams, are diagrams that show the bonding between atoms of a molecule, and the lone pairs of electrons that may exist in the molecule.
September 3, 2016 - WARNING! Long answer. > Here are the steps I follow when drawing a Lewis structure. 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ("Se"). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: ...
Transcript: This is Dr. B. Let's do the Lewis structure for SeF4 Lewis Structure. Se on the periodic table is in Group Six so it has six valence electrons. Fluorine, seven valence electrons but we have four of those. So let's multiply that out ... six plus twenty eight equals thirty-four total ...
A step-by-step explanation of how to draw the SeCl4 Lewis Dot Structure (Selenium Tetrachloride). Because Selenium is below Period (row) Two on the periodic ...
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity.
Example 1. What is the Lewis electron dot diagram for each element? a) aluminum b) selenium . Solution. a) The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: b) The valence electron configuration for selenium is 4s 2 4p 4.
Selenium element is located in Group 6A of the periodic table. This in dicates that it has 6 electron in its valence or outermost shell. The Lewis dot structure of Selenium shoulde be written with Se surrounded by six dots. These six dots don't have to be an all four sides but if you do then that is the conventional way of doing it.
The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows: ... Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca2+.
Draw the Lewis dot structure for Mg and Se. Covalent bonds. Expert Answers. Hover for more information. ... write the symbol for selenium, Se, and put an electron pair on the top, an electron pair ...
February 23, 2012 - ABOUT · Our Mission · Meet the Team · Partners · Press · Careers · Security · Status · Success Stories · Overview
Lewis Diagram For Seo3 A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) .
What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4.

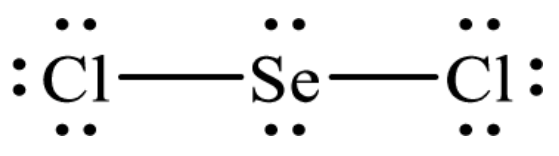















0 Response to "39 lewis dot diagram for selenium"
Post a Comment