I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c... Hey Guys, ​ I was wondering if there are some databases for molexular orbital diagrams of more unusual compounds like phosphaalkenes or sulfur nitrides. I wanted to include some in a presentation ​ thanks for any help!
[US/Buy] still searching for reasonable priced Orbits v1 & v3 open decks welcome to help keep cost more reasonable
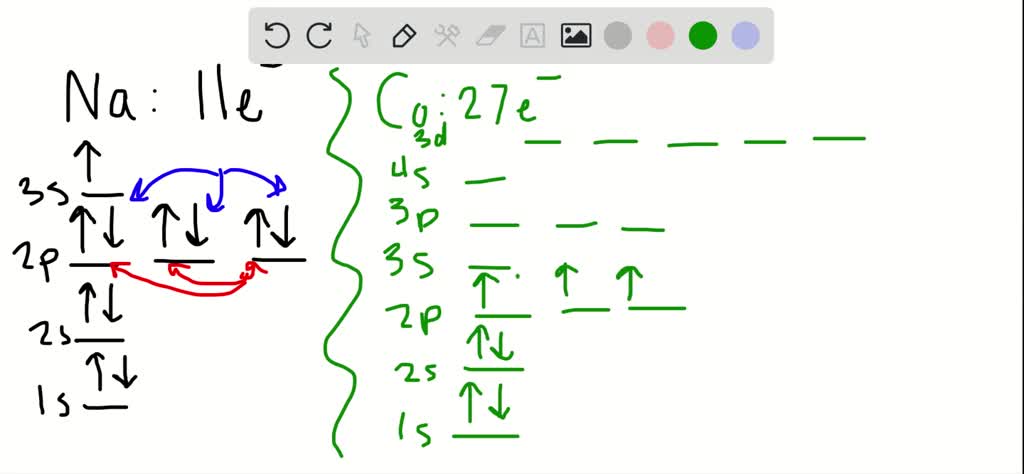
V5+ orbital diagram
Sorry if it's a dumb question, I'm having trouble understanding For group, there should be a fully filled s sublevel and two electron in the outermost p sublevel. Based on how covalent bonds form with singly filled orbitals, in group14, there are only 2 singly filled orbitals respectively, how can they form the expected 4 bonds? Or does the electron from s move to p to give 4 singly filled orbitals? If that is the case, why does this happen for no reason?
V5+ orbital diagram. For group, there should be a fully filled s sublevel and two electron in the outermost p sublevel. Based on how covalent bonds form with singly filled orbitals, in group14, there are only 2 singly filled orbitals respectively, how can they form the expected 4 bonds? Or does the electron from s move to p to give 4 singly filled orbitals? If that is the case, why does this happen for no reason? Sorry if it's a dumb question, I'm having trouble understanding

draw atomic orbital diagrams representing the ground state electron configuration for each of the 3

Crystal Imperfections of Industrial Vanadium Phosphorous ...

Paramagnetic vs Diamagnetic - Paired vs Unpaired Electrons - Electron Configuration

Schematic of the 'O2' molecular orbital diagram. The figure ...
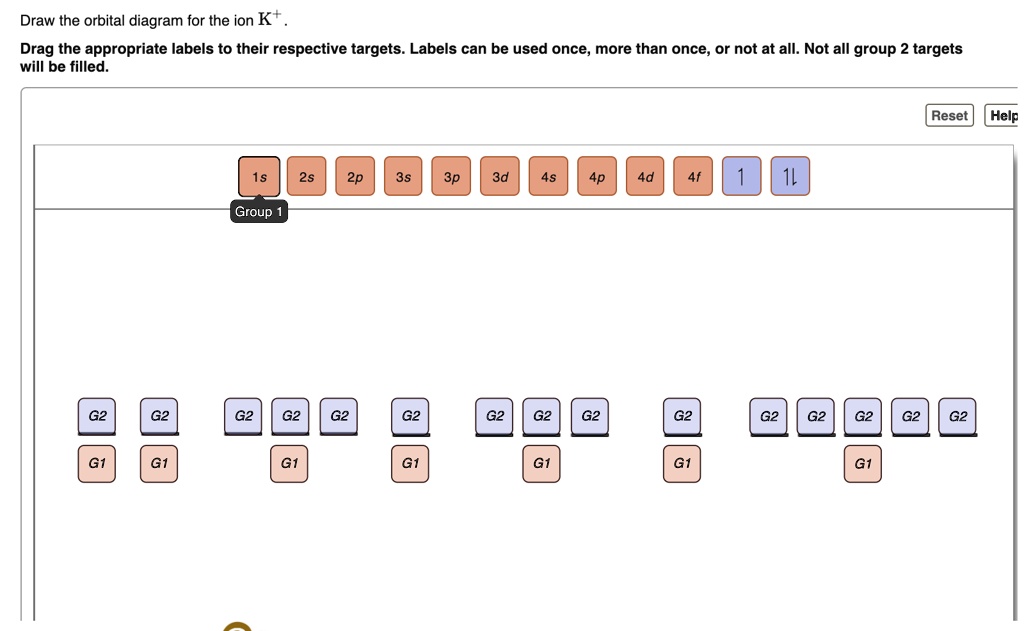
SOLVED:Draw the orbital diagram for the ion K + Drag the ...

A. Model Atom Bohr dan Mekanika Kuantum

Orbital diagram for zinc

Choose the correct orbital diagram for van... | Clutch Prep

Synthesis and optoelectronic properties of a promising ...

a) The temperature evolution of ESR spectra in the VOSb2O4 ...
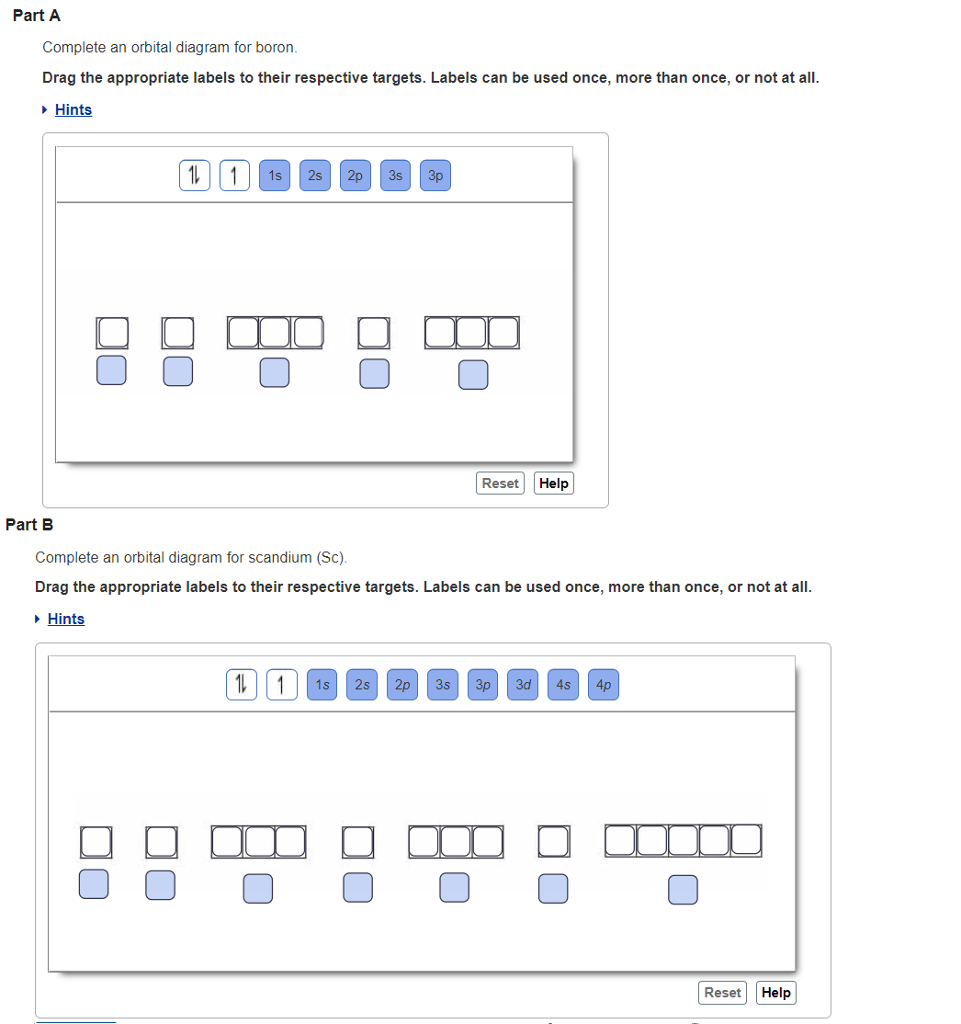
Solved Complete an orbital diagram for boron Drag the | Chegg.com

Molecular orbital energy-level diagram of anatase TiO2 ...

Orbital Diagram for Vanadium
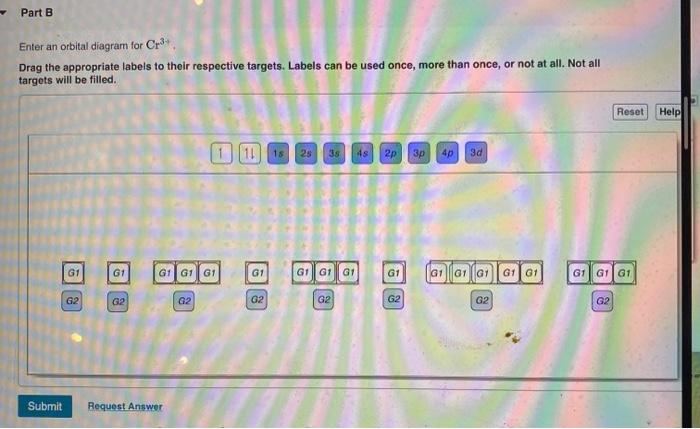
Solved Part A Enter an orbital diagram for V5+ Drag the ...
![arXiv:2106.10869v1 [cond-mat.mtrl-sci] 21 Jun 2021](x-raw-image:///ea88683934dc61c0cc986ac1f88a11d482b6fec11397ac3fc50e54a1053a22a8)
arXiv:2106.10869v1 [cond-mat.mtrl-sci] 21 Jun 2021

Felfi Fix Anorganik 2 | PDF

GOLONGAN TRANSISI Kelompok 10 OTW Inshaallah ...

Identify whether the ions are diamagnetic ... | Clutch Prep

1. Write orbital diagrams for each of these ions. *a. V5+ *b ...

3.1: Electron Configurations (Problems) - Chemistry LibreTexts

SOLVED:Write orbital diagrams for each ion and determine if ...
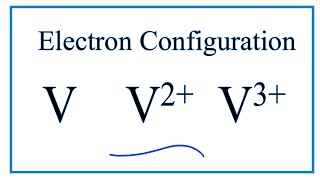
How to Write the Electron Configuration for V and V3+

What kinds of electron configurations can we get with ...

Schematic of the 'O2' molecular orbital diagram. The figure ...

Using VSEPR theory, predict the electron pair geometry and ...
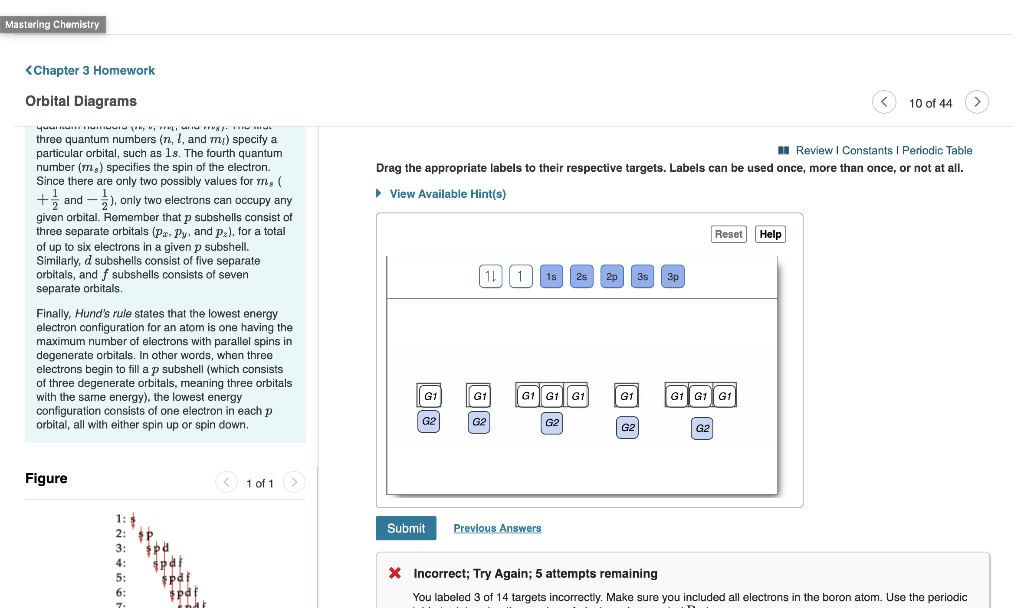
Solved Mastering Chemistry
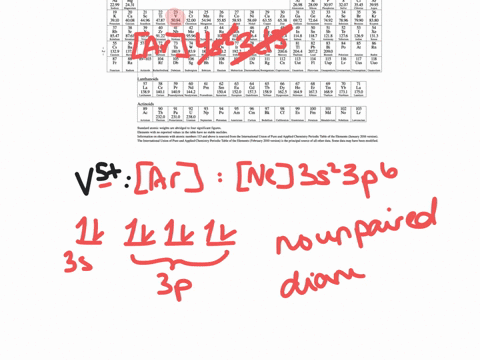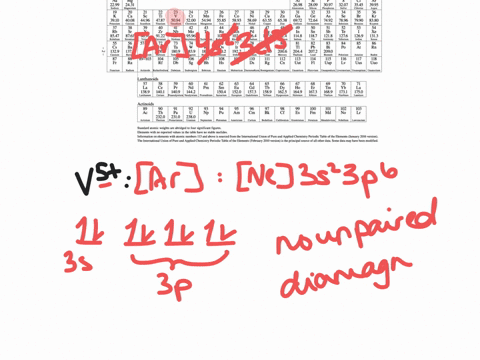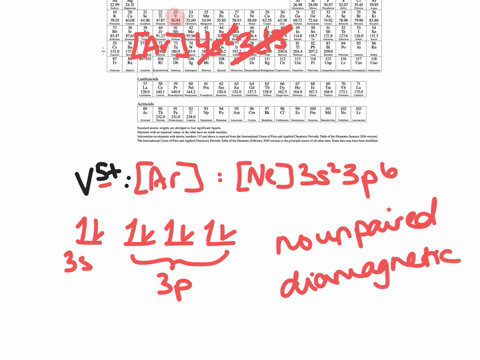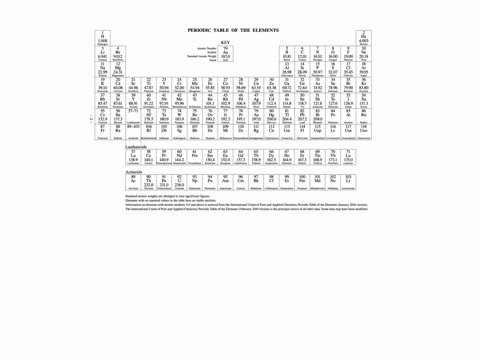
SOLVED:Write orbital diagrams for each ion and determine if ...

Untitled

Orbital diagram for vanadium ions - YouTube

2021 roadmap for sodium-ion batteries

Building Real-time Public Transport Tracking System on Azure ...

Prep101 - Chemistry 201 Term Test 1 Booklet Solutions

WebElements Periodic Table » Vanadium » properties of free atoms
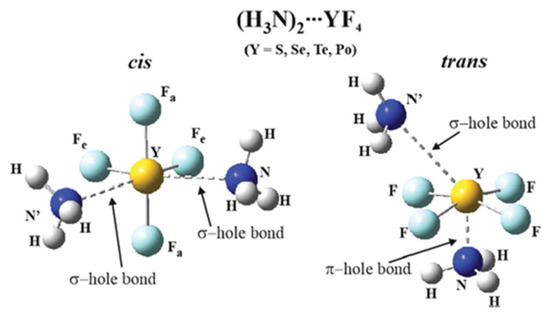
Molecules | Free Full-Text | Noncovalent Bonds through Sigma ...
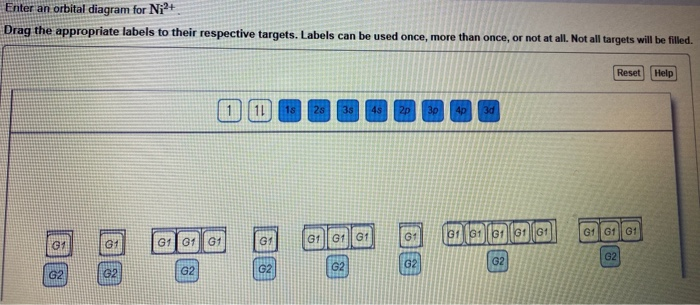
Solved I did'nt het this can someone explain me the process ...

UNIVERSITY OF THE WEST INDIES MONA, JAMAICA
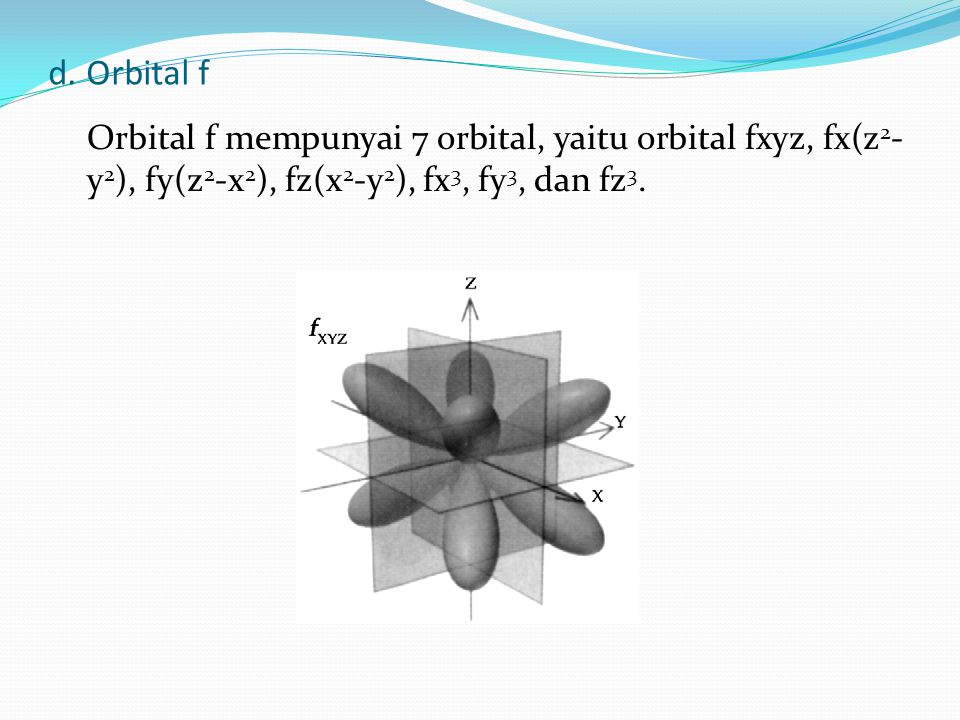
A. Model Atom Bohr dan Mekanika Kuantum - ppt download

Write orbital diagram to represent the ele... | Clutch Prep





























0 Response to "38 v5+ orbital diagram"
Post a Comment