Mn (Manganese) is an element with position number 25 in the periodic table. Located in the IV period. Melting point: 1244 ℃. Density: 7.44 g/cm 3 . Electronic configuration of the Manganese atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5. Jul 29, 2016 · The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram. Chemistry
To write the orbital diagram for the Manganese (Mn) first we need to write the electron configuration for just Mn. To do that we need to find the number of ...
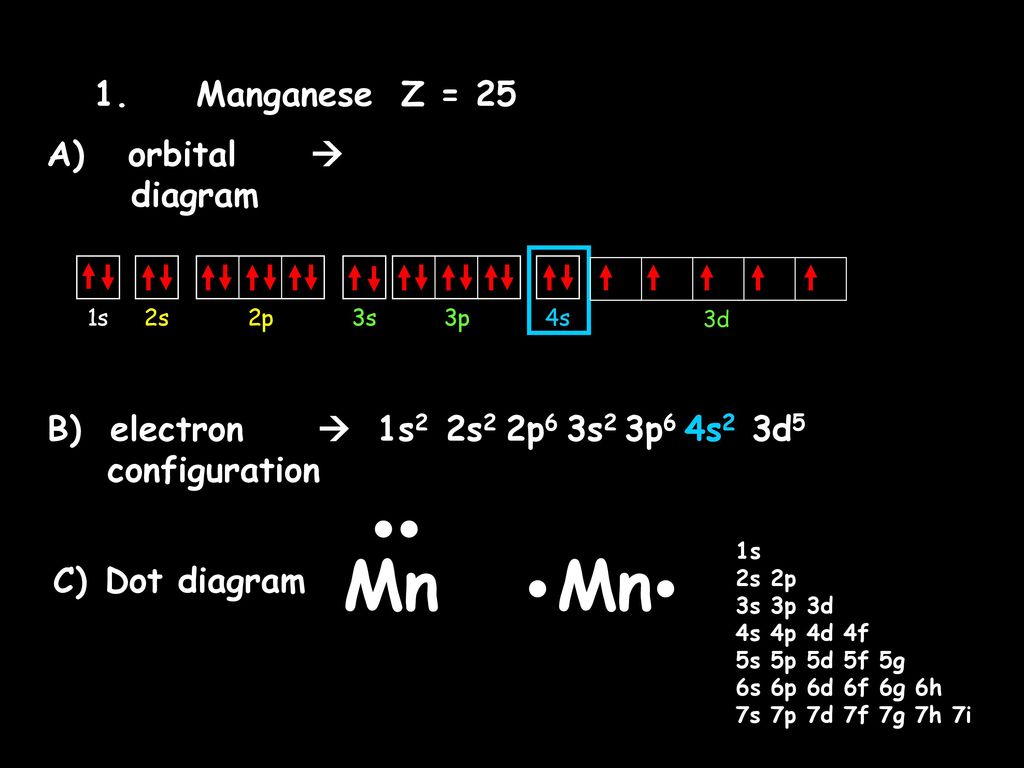
Orbital diagram for manganese
Manganese (Mn) has an atomic mass of 25. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ... Orbital Diagram. 1s ... Jan 04, 2022 · Atomic Orbital Diagram for Manganese (Mn) Manganese(Mn) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of manganese is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 2. The valency of the element is determined by electron configuration in the excited state.
Orbital diagram for manganese. Jan 04, 2022 · Atomic Orbital Diagram for Manganese (Mn) Manganese(Mn) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of manganese is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 2. The valency of the element is determined by electron configuration in the excited state. Manganese (Mn) has an atomic mass of 25. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ... Orbital Diagram. 1s ...

Orbital Diagrams, Electron Configurations, and Dot diagrams ...

How many unpaired electrons are in an atom of manganese ...
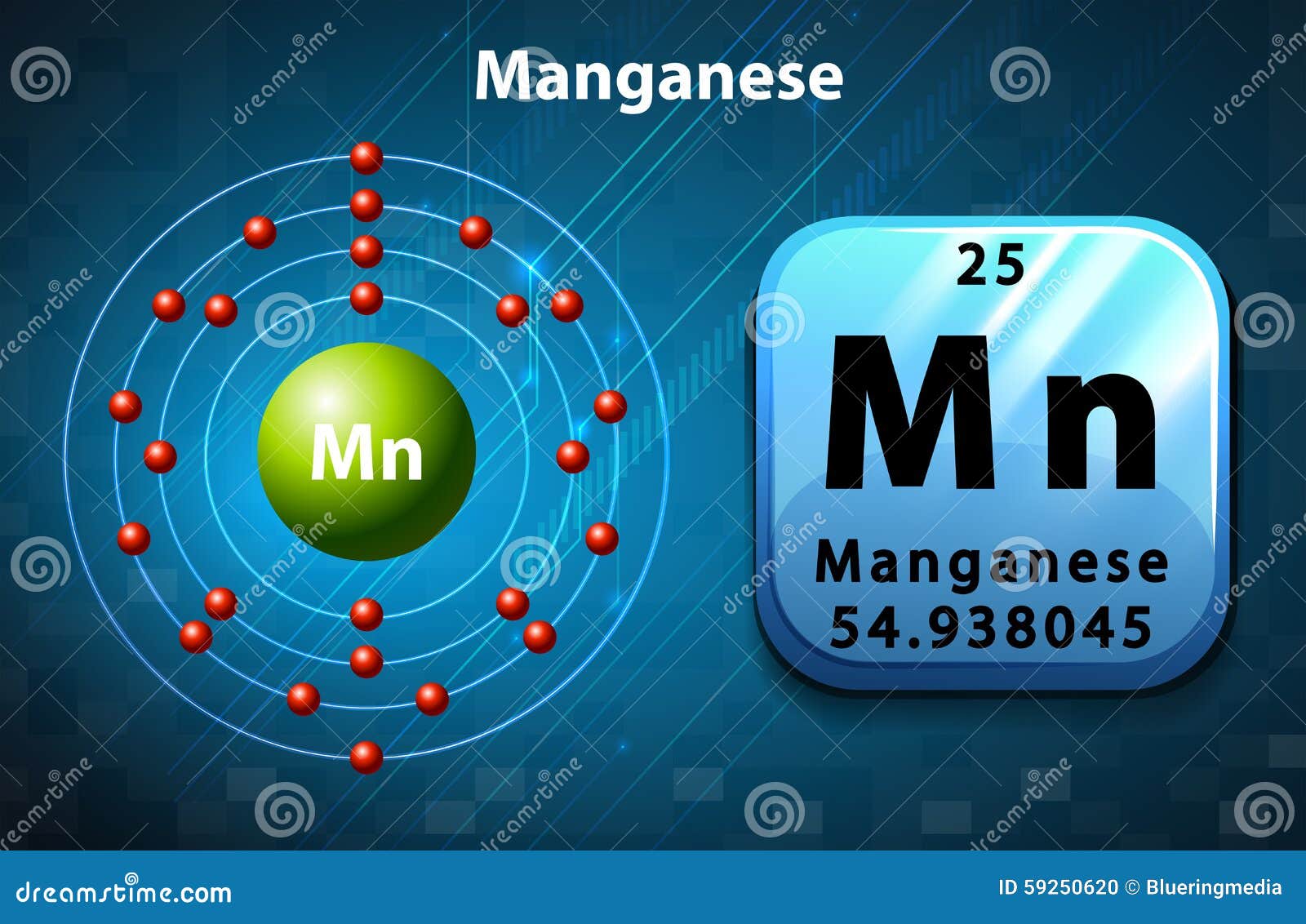
Periodic Symbol and Diagram of Manganese Stock Vector ...

how many electrons does a Mn atom have in its 3d subshell ...

Figure 4 from Molecular orbital ( SCF-Xc-SW ) theory of Fe 2 ...
Solved Manganese is found as MnO2 in deep ocean deposits ...

Electronic configuration of bromine?... | Clutch Prep

CHEM 1180: 22.6: Valence Bond Theory of Complexes

Electron Configurations & Activity 16 Intro. 3 Rules to ...

Chapter 7 Quantum Theory and the Electronic Structure of Atoms
![What is the hybridisation of [Mn(CN) 6] 3-? - Quora](https://qph.fs.quoracdn.net/main-qimg-6d08b96b2ac0ae5a5c0b54b6c4b3ecaa)
What is the hybridisation of [Mn(CN) 6] 3-? - Quora
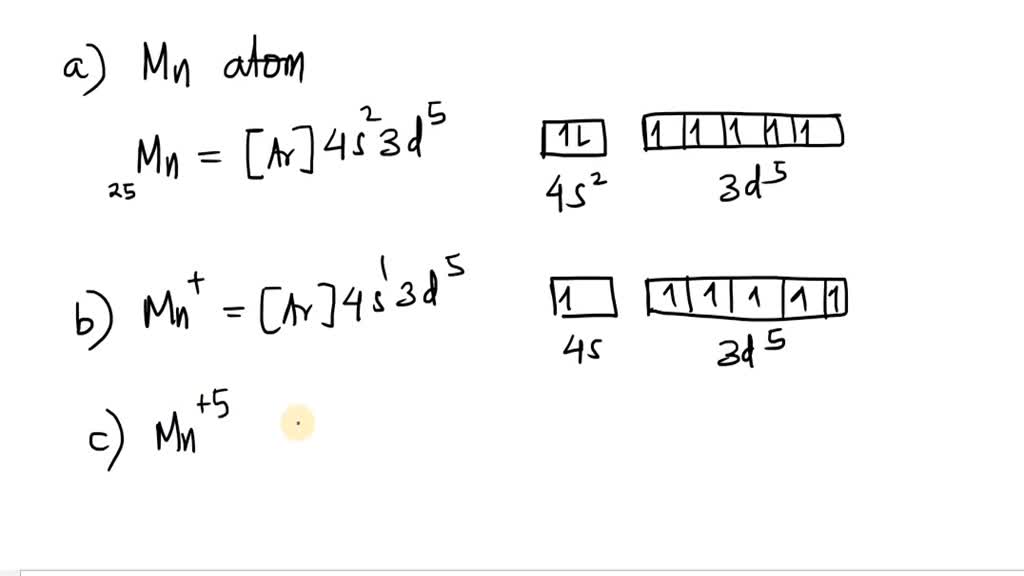
write the atomic orbital diagram for the 4 s and 3 d electrons in a a manganese atom b mathrmmn ion

Identifying the Correct Sequence of Orbital Diagrams to Show the Formation of the Mn²⺠Ion

In which of the following orbital diagrams are both Pauli ...
![Exchange coupling through diamagnetic [Fe(CO) 4 ] 2 ...](https://pubs.rsc.org/image/article/2011/DT/c0dt01221a/c0dt01221a-f2.gif)
Exchange coupling through diamagnetic [Fe(CO) 4 ] 2 ...
![Which element has the noble gas configuration [Ar]4s23d ...](https://img.homeworklib.com/questions/6e209250-77e8-11ea-a268-ffa2087b6b94.png?x-oss-process=image/resize,w_560)
Which element has the noble gas configuration [Ar]4s23d ...
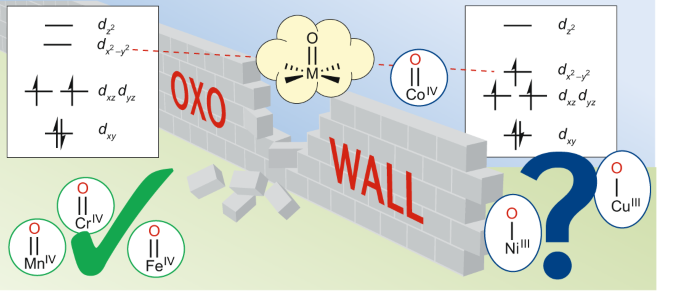
Iron and manganese oxo complexes, oxo wall and beyond ...
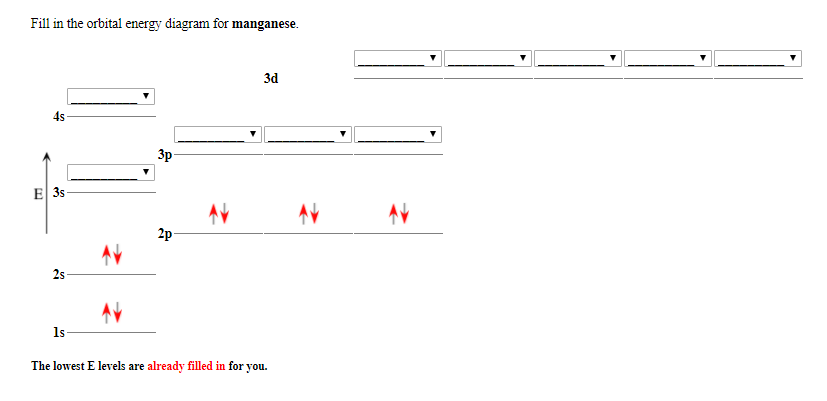
Solved Fill in the orbital energy diagram for manganese. 3d ...
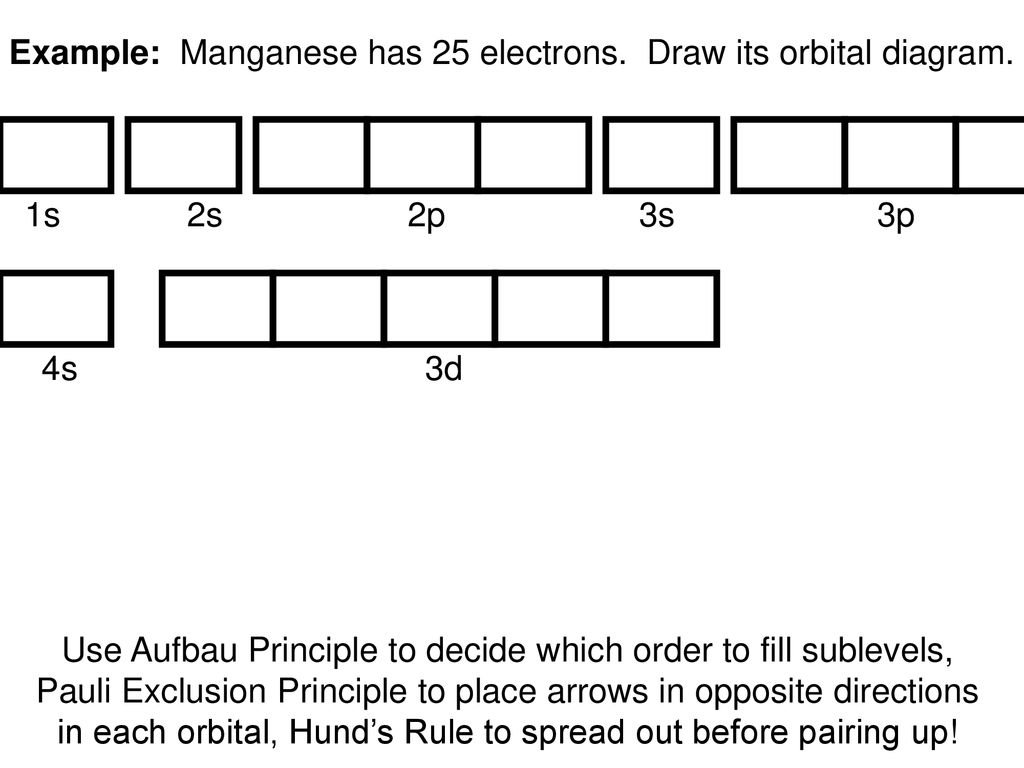
Orbital Diagrams. - ppt download

CHM 1025C On-Line Module 3 Practice Final
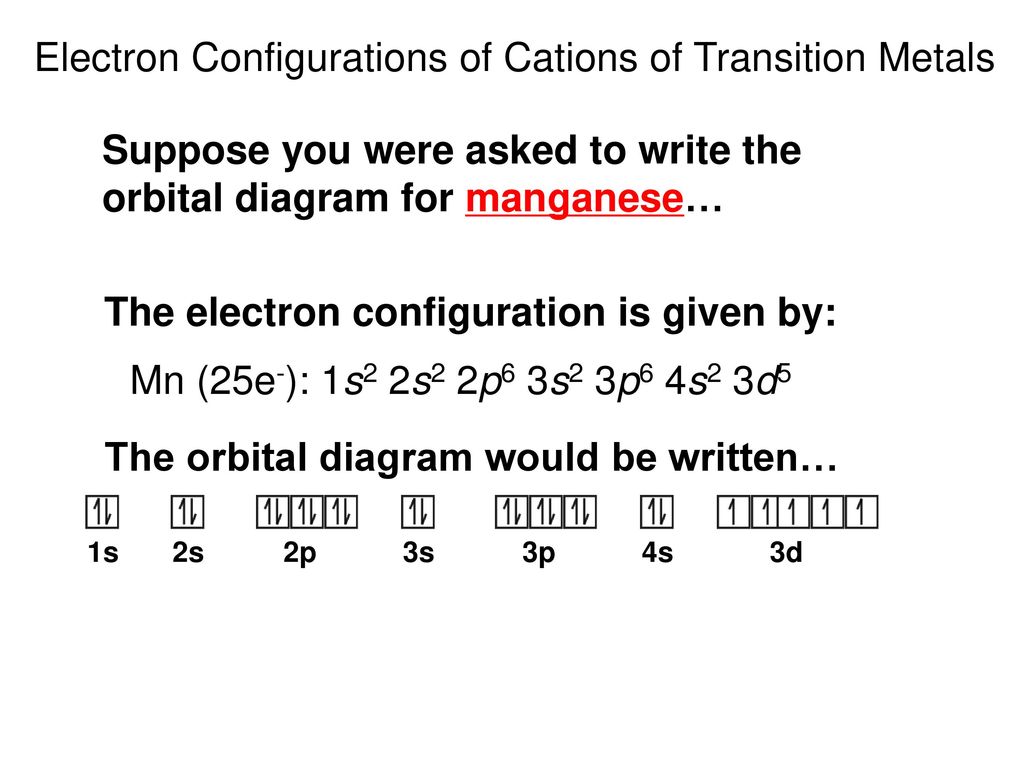
Periodic Relationships Among the Elements - ppt download

Group 7 : Manganese Chemistry

Orbital energy diagrams showing the possible ways that Mn(IV ...
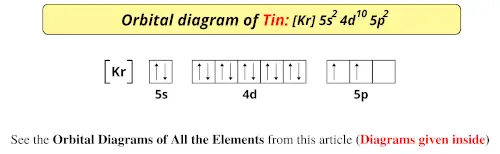
Orbital Diagram of All Elements (Diagrams given Inside)

The electronic configuration of the `Mn^(4+)` ion is -

Introductory Chemistry 3 rd Edition Nivaldo Tro Chapter

Answered: II Electron Configuration and Orbital… | bartleby

What Is Zinc Electron Configuration | Know It Info

8. ELECTRON CONFIGURATIONS AND PERIODICITY - Teacher

WebElements Periodic Table » Manganese » properties of free atoms

The electronic structures of manganese oxide minerals
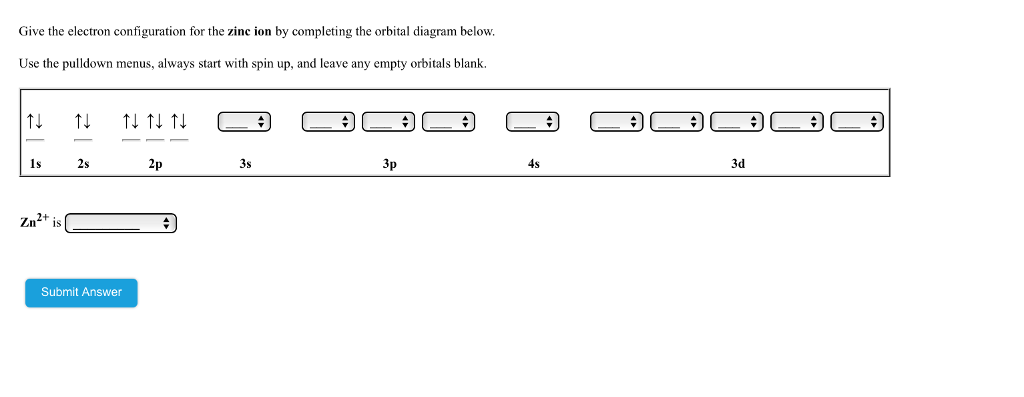
Solved Give the electron configuration for manganese by ...
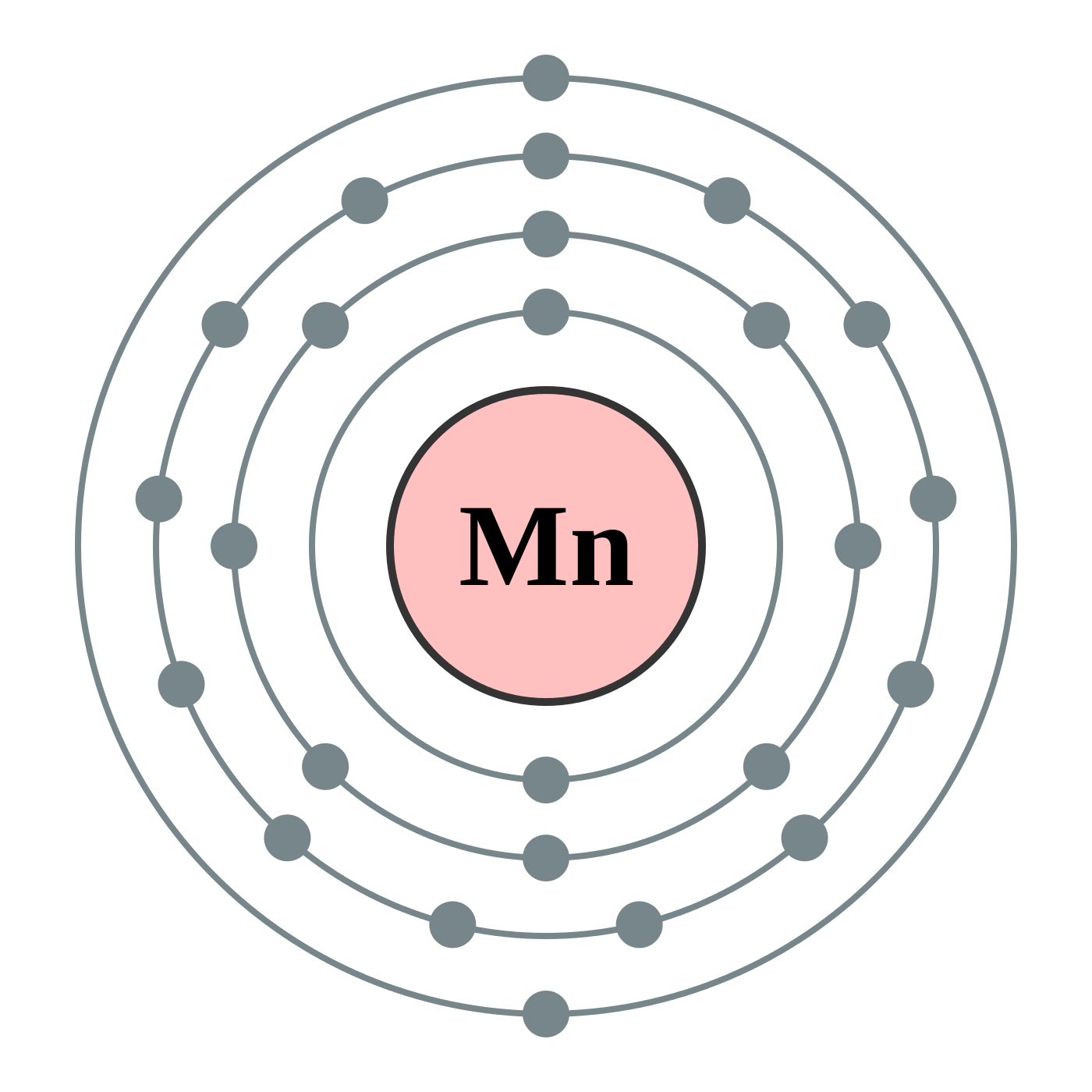
Manganese Electron Configuration - Dynamic Periodic Table of ...
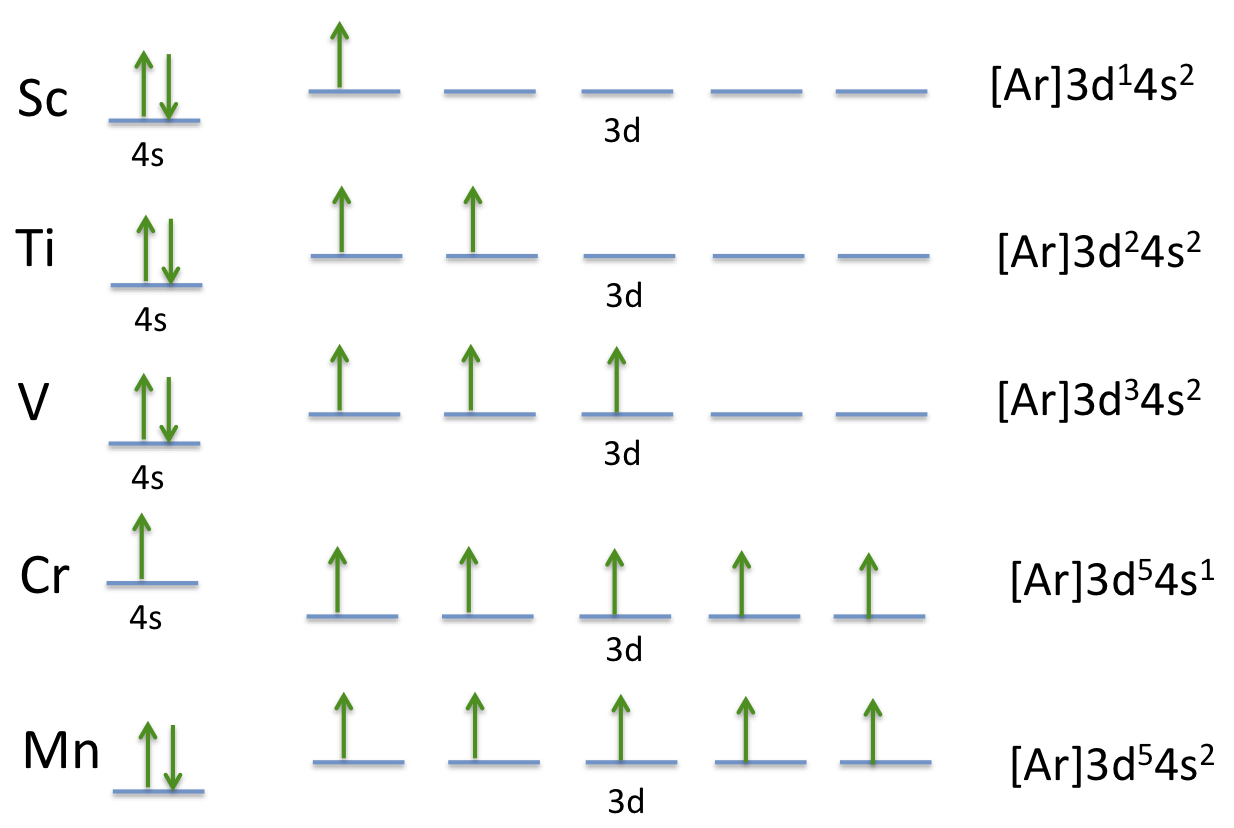
Chapter 2.7: Electronic Structure of the Transition Metals ...

How do you draw the electron configuration... | Clutch Prep

Electron Configuration and Orbital Diagram
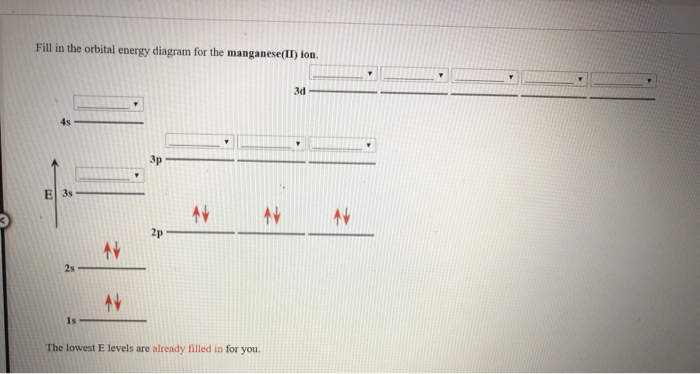
Solved Fill in the orbital energy diagram for the | Chegg.com












![Exchange coupling through diamagnetic [Fe(CO) 4 ] 2 ...](https://pubs.rsc.org/image/article/2011/DT/c0dt01221a/c0dt01221a-f2.gif)
![Which element has the noble gas configuration [Ar]4s23d ...](https://img.homeworklib.com/questions/6e209250-77e8-11ea-a268-ffa2087b6b94.png?x-oss-process=image/resize,w_560)


















0 Response to "37 orbital diagram for manganese"
Post a Comment