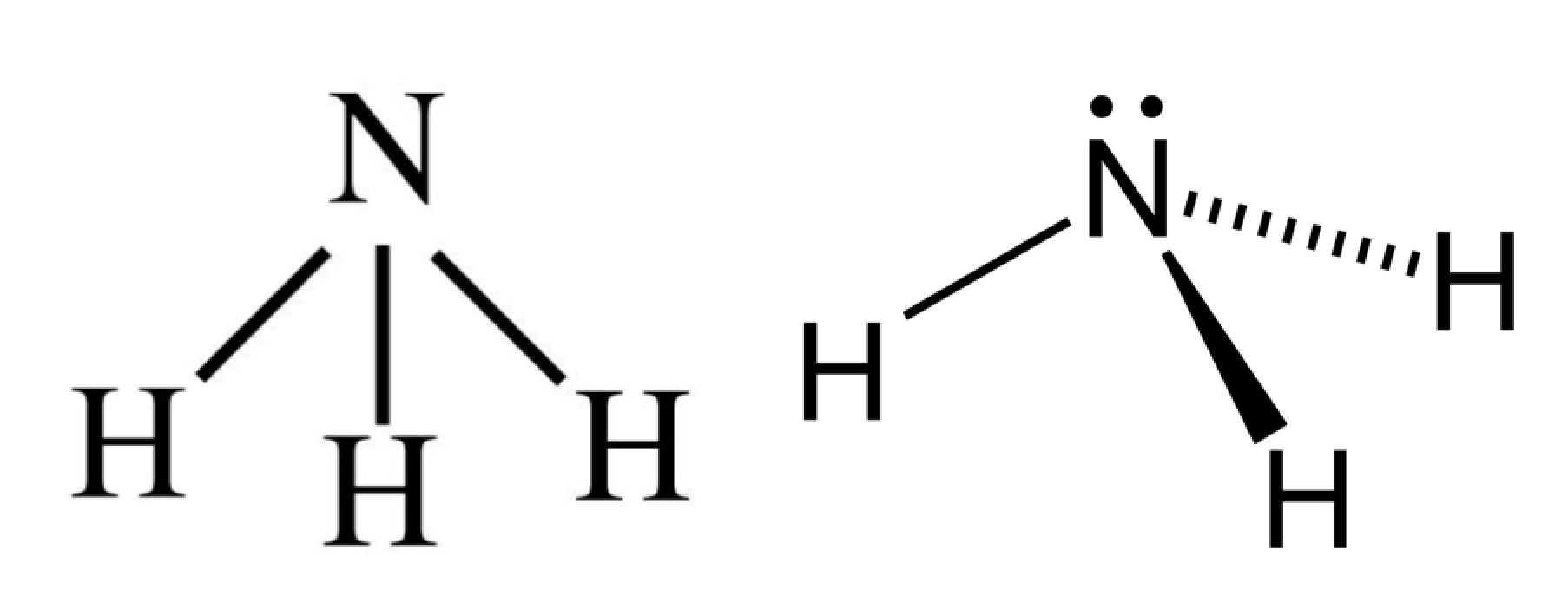
39 the ammonia molecule in the diagram has the observed bond orientation because ...
The central carbon in such compounds is sp-hybridized (it has only two bonding partners), and the double bond array is linear as a result. Since the π-bonds of allenes are orthogonal, the planes defined by the end carbon substituents are also orthogonal. As shown in the following diagram, the overall configuration of allenes resembles that of an elongated tetrahedron. An … Answer (1 of 5): NH3 HAS ONLY ONE LONE PAIR H2O HAS TWO LONE PAIRS The two lone pairs present in the oxygen atom of H2O molecule repels the two bond pairs. This repulsion is stronger than the repulsion between the lone pair and the three bond pairs on the nitrogen atom. Since the repulsions on...
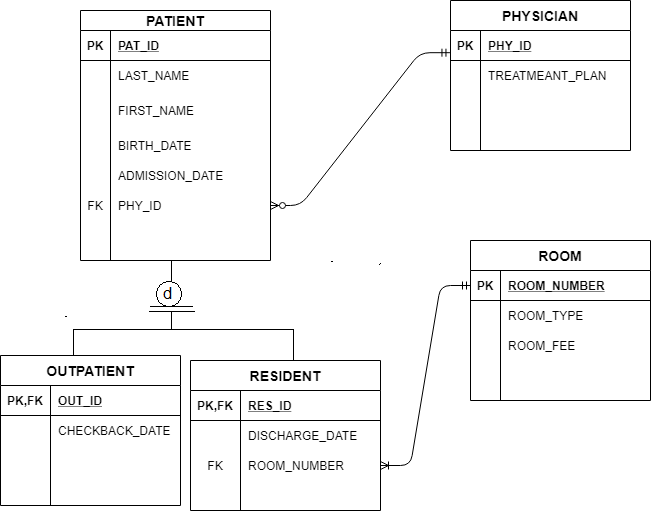
Molecular geometry. The theory of valency which we have been developing is known as valence bond theory.One further feature of this theory is that it may be used to predict (or in some cases, rationalize) the observed geometries of molecules By the geometry of a molecule we mean the relative arrangement of the nuclei in three-dimensional space.
The ammonia molecule in the diagram has the observed bond orientation because ...
For example, the orientation of the two O–H bonds in a water molecule (Figure 5.8) is bent: one end of the molecule has a partial positive charge, and the other end has a partial negative charge. In short, the molecule itself is polar. The polarity of water has an enormous impact on its physical and chemical properties. If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. Some three-atom molecules also have straight-line geometry. For example: Notice that, in the Lewis structure of these molecules, the central atom(s) bonds with only two other atoms and has no unshared electrons. Dec 17, 2021 · In the diagram given alongside, one of the oxygen atoms from the original \({{\rm{O}}_2}\) molecule shown in black has contributed both of its non bonded pair of electrons to the blue oxygen atom. The blue oxygen atom has not contributed any of its existing electrons to this bonding, so this is a coordinate covalent bond (dative bond).
The ammonia molecule in the diagram has the observed bond orientation because .... The most common catalyst in the Haber-Bosch process for the hydrogenation of dinitrogen (N 2) to ammonia (NH 3) is an iron surface promoted with potassium cations (K +), but soluble iron complexes have neither reduced the N-N bond of N 2 to nitride (N 3-) nor produced large amounts of NH 3 from N 2.We report a molecular iron complex that reacts with N 2 and a potassium reductant to give a... Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space ().A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. When the pH is above 7.2, some free NH3 remains and this increases with increasing pH (1). The equilibrium for these chemical species can be expressed by the following: NH3 + H2O <=> NH4OH <=> NH4+ + OH- (1). The reaction between ammonia and water is reversible ( ammonium hydroxide reverting to ammonia and water ) (2). The ammonia molecule in the diagram has the observed bond orientation because from BIO at Western Michigan University. The ammonia molecule in the diagram has the observed bond orientation because All of the above (N has 7 protons in its nucleus.
Ammonia is known to behave as a weak base since it combines with many acids to form salts. For example, when it is reacted with hydrochloric acid, ammonia is converted into ammonium chloride.All the salts that are produced from such acid-base reactions are known to contain the ammonium cation, denoted by NH 4 +.It is interesting to note that ammonia also exhibits weak acidic qualities and can... The ammonia molecule (NH3) in the diagram has the observed bond orientation because...a. N has four pairs of electrons in the valence shell. b. N has 7 protons in its nucleus. c. electrons repel one another. d. All of the above. e. None of the above. The Fischer projection formula of meso-tartaric acid has a plane of symmetry bisecting the C2–C3 bond, as shown on the left in the diagram below, so this structure is clearly achiral. The eclipsed orientation of bonds that is assumed in the Fischer drawing is, however, an unstable conformation, and we should examine the staggered conformers ... Academia.edu is a platform for academics to share research papers.
Academia.edu is a platform for academics to share research papers. Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR theory, molecules try to acquire a shape which would minimize the repulsion exhibited by the electron clouds present, that is, between the bonding (shared in a bond) and non-bonding (lone pair) electrons. The molecule has a single bond. The molecule has no electrons in antibonding orbitals. The molecule is as stable as molecules with bond orders of two and three. Two side-by-side p orbitals combine to form pi bond and pi antibond orbitals; therefore, the bond order is 1. Chem 302. The Molecular Orbitals of Ammonia. Determing the electronic structure of ammonia will introduce the new ideas of degenerate orbitals and degenerate axes. It is important to understand these concepts because of the large number of molecules that have point groups such as C 3v or D 3h. To determine the MO's of ammonia.
The Ammonia Molecule In The Diagram Has The Observed Bond Orientation Because...1jzgte Mk3 Supra Tachometer Wiring Diagram; Trrs Plug Wiring; Kenwood Kdc 1011s Wiring Diagram; Sensi St55 Wiring Diagram; 1999 Oldsmobile Intrigue Radio Wiring Diagram; Quadrajet Vacuum Ports Diagram; Baldor Cl3608tm Wiring Diagram; Tach Wiring Diagram For A 81...
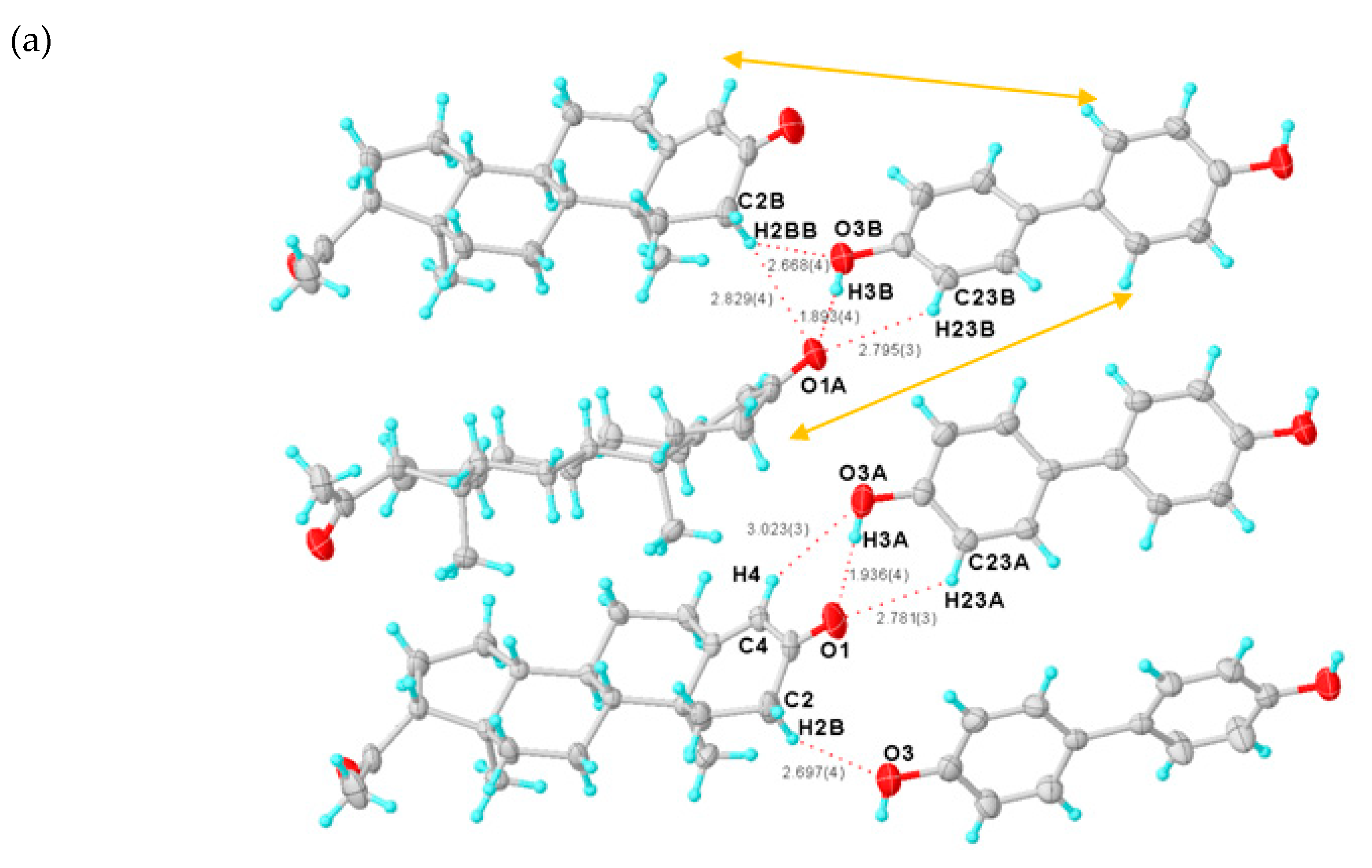
Figure 27 illustrates the hydrogen bonding as observed in crystalline ammonia. The hydrogen bonds are longer than those in ice and are non-linear. Although each ammonia molecule forms hydrogen bonds with six neighbors in the crystal, only two ammonia molecules are shown here.
(a) Each CO bond has a bond dipole moment, but they point in opposite directions so that the net CO 2 molecule is nonpolar. (b) In contrast, water is polar because the OH bond moments do not cancel out. The OCS molecule has a structure similar to CO 2, but a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule is...
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq...
Covalent Bonds hold atoms together because they....an atoms atomic number is 7. Its valence is most likely. 3. By making two covalent bonds, an O atom (w/ 8 protons) fills its valence shell...In a double covalent bond, a carbon atom shares.. electrons in two orbitals. The ammonia molecule in the diagram has the observed bond orientation...
These diagrams tell us that the F 2 molecule has a single bond, the CO 2 molecule has two double bonds, and the HCN molecule has one single bond plus one triple bond.. There are two main methods for constructing such diagrams. Part I of this document describes the smart way to do it. This involves counting the bonding and antibonding electrons, so we call it the BABE method.
Ice. Ice (H 2 O) n Ice is hydrogen-bonded network of water.. Water is formed when two atoms of hydrogen bond covalently with an atom of oxygen. The molecule is not linear because the non-bonded electrons (2 unshared pairs) of oxygen repel each other; this leads to a non-linear (or bent-angular) structure.
The ammonia molecule in the diagram has the observed bond orientation because...A. N has four pairs of electrons in the valence shell. B. electrons repel one another. C. N has 7 protons in its nucleus. D. All of the above. E. None of the above.
The ammonia molecule in the diagram has the observed bond orientation because… All of the above (N has 7 protons in its nucleus, electrons repel one another, N has four pairs of electrons in the valence shell)
Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR theory, molecules try to acquire a shape which would minimize the repulsion exhibited by the electron clouds present, that is, between the bonding (shared in a bond) and non-bonding (lone pair) electrons.
The ammonia molecule in the diagram has the observed bond orientation because...A. N has four pairs of electrons in the valence shell. B. electrons repel one another. C. N has 7 protons in its nucleus. D. All of the above. E. None of the above.
The binding of urea to the active site of urease has not been observed. ... A hydrogen bonds to one of the nitrogen atoms, breaking its bond with carbon, and releasing an NH 3 molecule. Simultaneously, the bond between the oxygen and the 6-coordinate nickel is broken. This leaves a carbamate ion coordinated to the 5-coordinate Ni, which is then displaced by a water …
Covalent bonds hold atoms together because they... fill shells without giving atoms much charge...The ammonia molecule in the diagram has the observed bond orientation b/c? all of the above...an electrically neutral molecule has the formula C3H4O2N. If the carbon atoms from the usual number of bonds, how many covalent bonds will each...
Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase.The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those …
The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above
For ammonia, NH 3, the symmetric bending vibration is observed as two branches near 930 cm −1 and 965 cm −1. This so-called inversion doubling arises because the symmetric bending vibration is actually a large-amplitude motion known as inversion , in which the nitrogen atom passes through the plane of the three hydrogen atoms, similar to ...
15.03.2020 · Because of their intriguing mechanical and electronic properties, together with the richness of elemental composition and chemical decoration, MXenes are poised to provide a new 2D nanoplatform for advanced optoelectronics. This comprehensive review, intended for a broad multidisciplinary readership, highlights the state-of-the-art progress on MXene theory, materials …
The observed structure of the borane molecule, BH 3, suggests sp 2 hybridization for boron in this compound. The molecule is trigonal planar, and the boron atom is involved in three bonds to hydrogen atoms (Figure7.5.7). Figure 7.5.7. BH 3 is an electron-deficient molecule with a trigonal planar structure.
Nitrogen has a total of 7 protons (its atomic number is 7) in its nucleus. Explanation: The shape and the bond orientation of molecules and ions are both ...1 answer · Top answer: Answer:Nitrogen has four pairs of electrons: 3 bonds and 1 lone pair in the valence shell;Electrons repel one another based on the VSEPR theory;Nitrogen ...
Because ammonia is given off when amino acids are metabolized to yield energy but not when sugars and fats are metabolized, you would expect more nitrogenous waste to be excreted. Choice (c) is incorrect because amino acids can be converted into pyruvate and acetyl CoA and used to generate energy. If more amino acids are consumed than are used, the body will not …
Bonding configurations are readily predicted by valence-shell electron-pair repulsion theory, commonly referred to as VSEPR in most introductory chemistry texts. This simple model is based on the fact that electrons repel each other, and that it is reasonable to expect that the bonds and non-bonding valence electron pairs associated with a given atom will prefer to be as far apart as possible.
Dec 17, 2021 · In the diagram given alongside, one of the oxygen atoms from the original \({{\rm{O}}_2}\) molecule shown in black has contributed both of its non bonded pair of electrons to the blue oxygen atom. The blue oxygen atom has not contributed any of its existing electrons to this bonding, so this is a coordinate covalent bond (dative bond).
If a molecule contains only two atoms, those two atoms are in a straight line and thus form a linear molecule. Some three-atom molecules also have straight-line geometry. For example: Notice that, in the Lewis structure of these molecules, the central atom(s) bonds with only two other atoms and has no unshared electrons.
For example, the orientation of the two O–H bonds in a water molecule (Figure 5.8) is bent: one end of the molecule has a partial positive charge, and the other end has a partial negative charge. In short, the molecule itself is polar. The polarity of water has an enormous impact on its physical and chemical properties.



















0 Response to "39 the ammonia molecule in the diagram has the observed bond orientation because ..."
Post a Comment