37 exothermic potential energy diagram
Start studying Exothermic Potential Energy Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools. A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
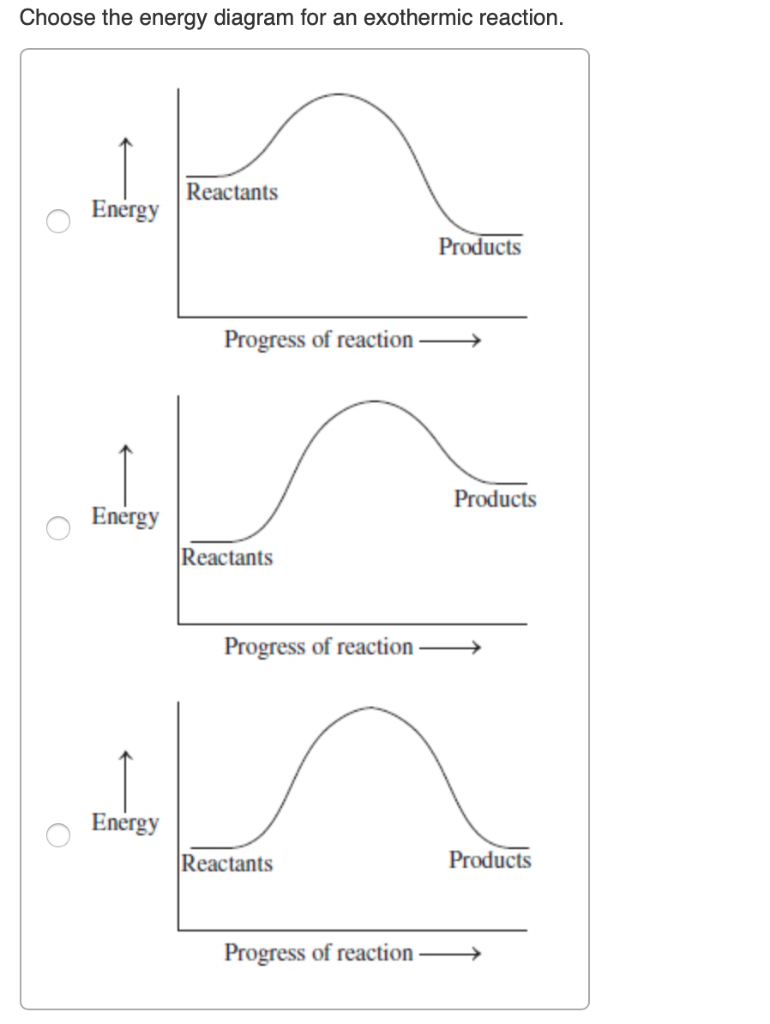
You can start with a generic potential energy diagram for an exothermic reaction. A The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. There is a greater difference in energy between the reactants and products. Because the reactants have more energy than the products they are less stable.

Exothermic potential energy diagram
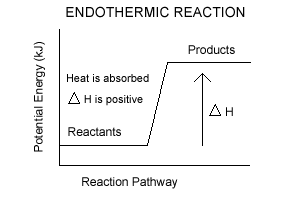
If the initial state has a higher potential energy than the final state, the process is exothermic. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b. Start studying Potential Energy Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Exothermic potential energy diagram. the energy released (∆H) Diagram 3 d) a spontaneous exothermic reaction. Diagram 2 depending on the values but looks quite small. To be spontaneous needs to be ≤ 20kJ 2. Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and Potential energy diagrams Consider an arbitrary potential energy shown schematically below. There are a number of important qualitative features of the behavior of the system that can be determined by just knowing this curve. The first thing to notice is that since the kinetic energy Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn , as well as DG 1 * and DG 2 * for the first and second activation energies, respectively. diagram. The vertical axis on this diagram represents the energy level and the horizontal axis represents the progress of the reaction from reactants to products. Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between
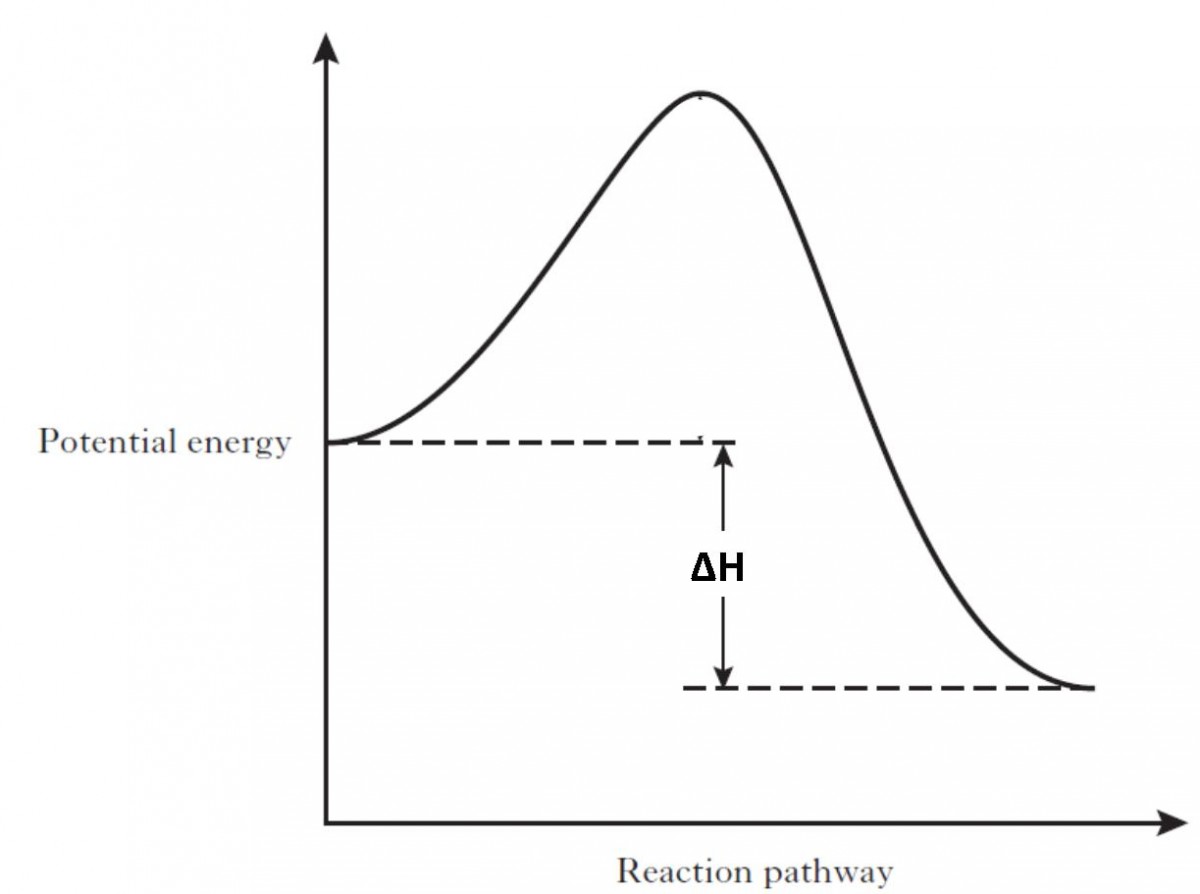
An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. Exothermic ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams.... Energy level diagram for an exothermic reaction is shown below. Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Look at the two exothermic reactions whose potential energy diagrams are represented in figures A and B below, and notice the activation energy marked in each. When a reaction has a low activation energy, like in figure A above, most of the reactant molecules have sufficient kinetic energy to react, and the reaction will most likely be rapid (a ...
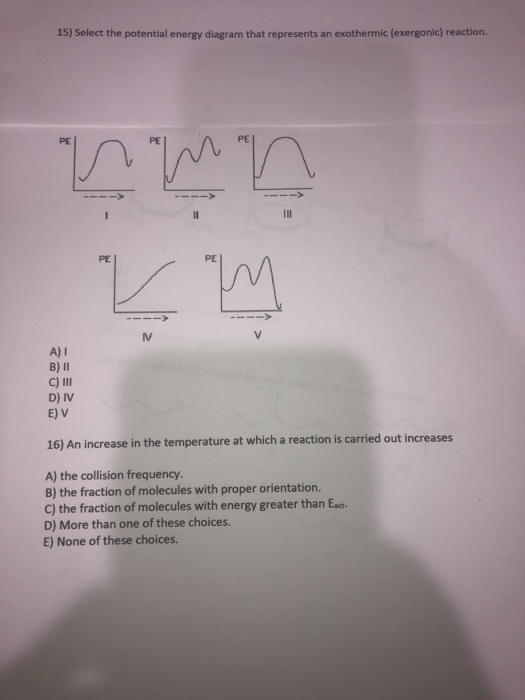
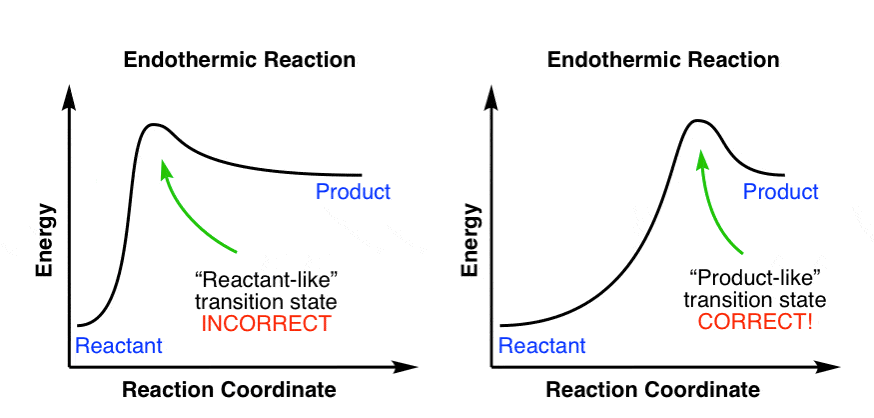
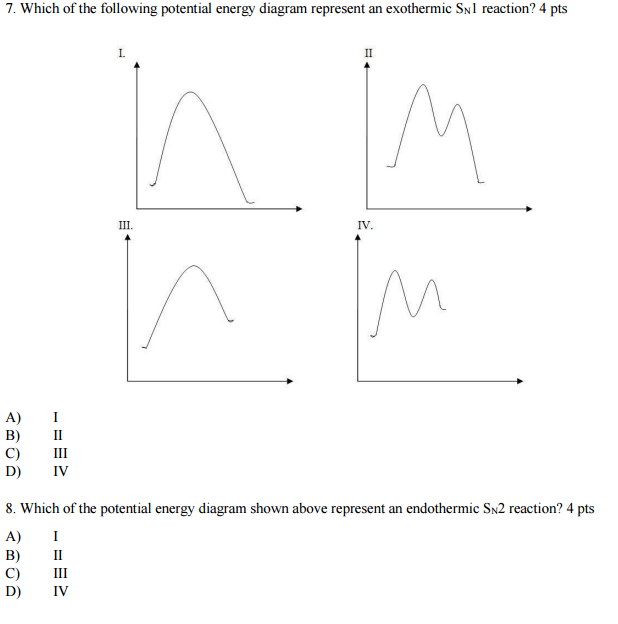
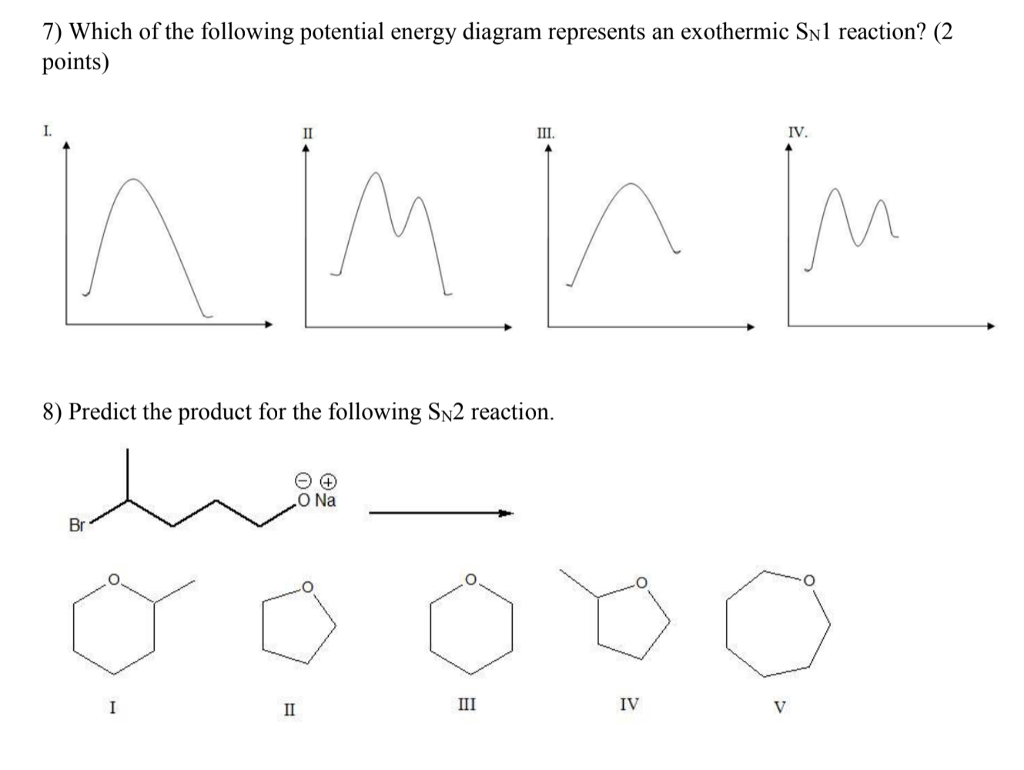
Question: Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential ... Exothermic and Endothermic Potential Energy Diagrams There are two types of potential energy diagrams. These two types center on the difference between the energies of the reactants and products. Consider the figure below. An endothermic reaction is shown on the left, and an exothermic reaction is shown on the right.. Q. The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. The reactants are represented by the horizontal line at the far left of the graph ... Jul 9, 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
An exothermic reaction is one in which heat energy is given out. The products must have less energy than the reactants because energy has been released. This can be shown by a potential energy...
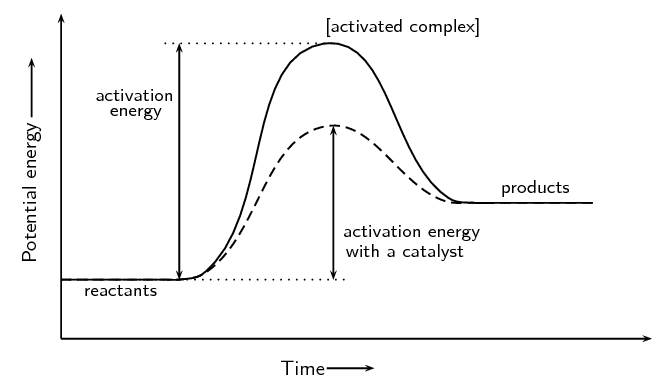
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants), through stage 2 (activated complex to), to stage 3 (products ...
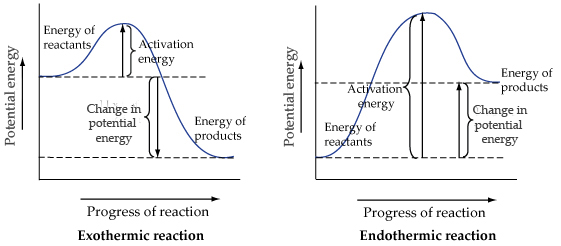
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
ENERGY DIAGRAM. • A = POTENTIAL ENERGY OF REACTANTS. • B = ACTIVATION ENERGY. • ENERGY REQUIRED TO START THE REACTION. • C = ACTIVATION ENERGY (REVERSE.11 pages
The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction.
Feb 23, 2012 — Potential energy diagrams show how the potential energy changes during reactions from reactants and products. · Exothermic reactions have a ...
Potential Energy Diagram Worksheet. Adrianne Lefevre. November 15, 2021. Potential Energy Diagram Practice Endothermic And Exothermic Reactions Potential Energy Energy Activities Exothermic Reaction. Chemistry 30 Chemical Kinetics Potential Energy Diagrams Revisited Chemistry Education Teaching Chemistry Chemistry.
We have to draw a potential energy diagram for the given reaction: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O (g) + 2219.9 kJ. This reaction is the combustion of propane (C 3 H 8).. Combustion is a reaction where a compound burns in oxygen to produce carbon dioxide (CO 2) and water (H 2 O).. To draw the potential energy diagram for this reaction, we have to first determine whether this ...
Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...
Answer (1 of 4): And yet again, a lazy, stupid student who just pastes their homework question into Quora, not even politely asking for help and referring to a diagram that ISN'T supplied. No wonder you don't know the answer. Yes, I'm being honest, and cruel, but I hope you can see that you are...
Nov 23, 2021 · Review. Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
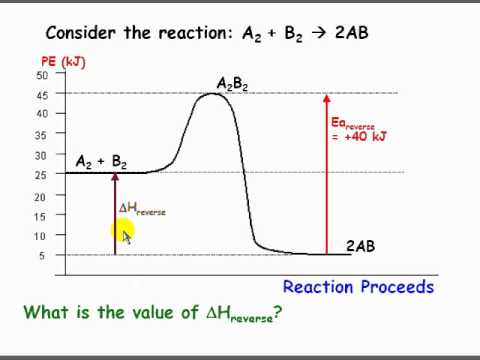
The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
Jul 9, 2019 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer--
How does a catalyst affect a potential energy diagram? ... Exothermic reaction is a chemical reaction in which energy is released into environment in form of heat. For example of exothermic reaction. Is a combustion reaction such as lighting a candle whereby oxygen react with wax to produce carbon (iV) oxide, water and heat. ...
Start studying Potential Energy Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
If the initial state has a higher potential energy than the final state, the process is exothermic. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.





















0 Response to "37 exothermic potential energy diagram"
Post a Comment