36 endothermic potential energy diagram
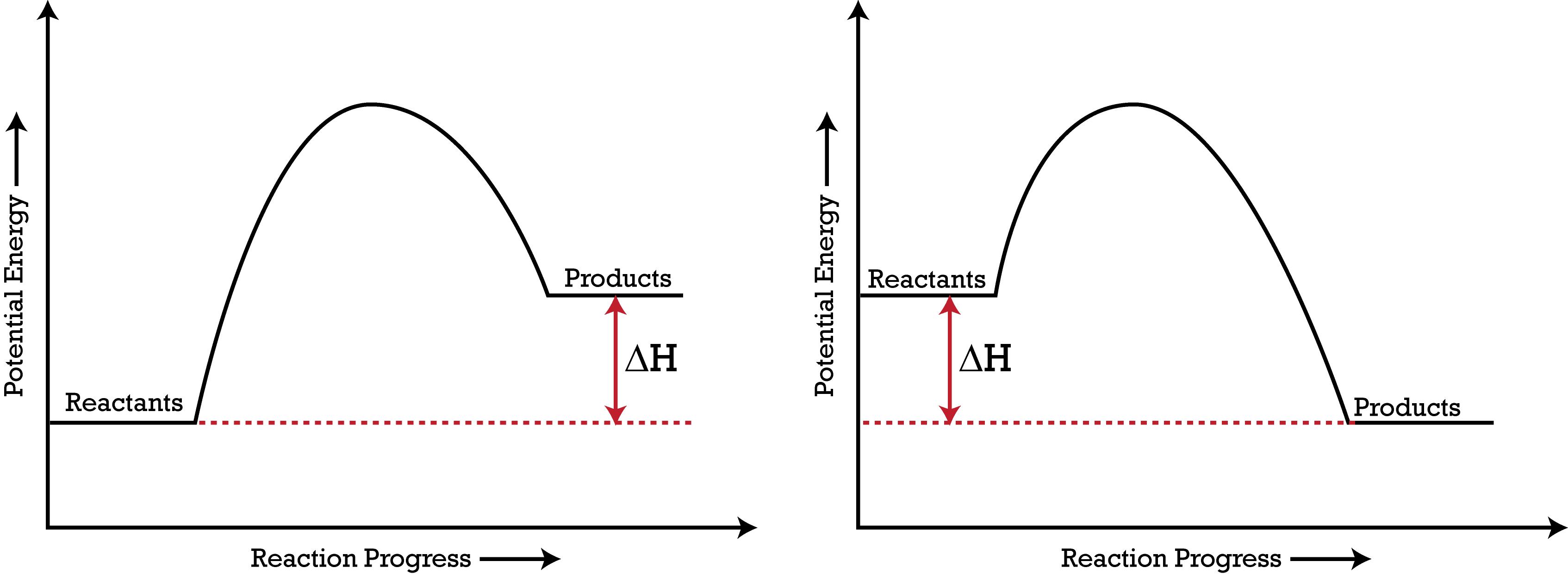
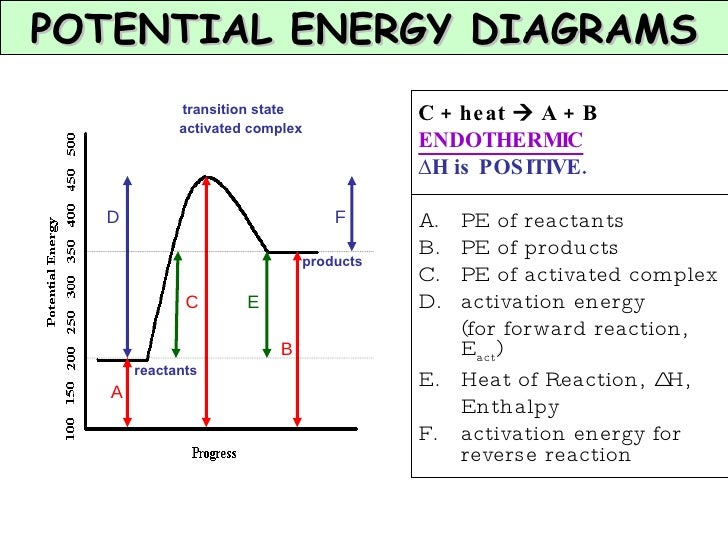
52 Sketch the potential energy diagram for an endothermic chemical reaction that shows the activation energy and the potential energy of the reactants and the potential energy of the products. Answer--> 1/04. 16 Which statement best explains the role of a catalyst in a chemical reaction? Exothermic and Endothermic Potential Energy Diagrams There are two types of potential energy diagrams. These two types center on the difference between the energies of the reactants and products. Consider the figure below. An endothermic reaction is shown on the left, and an exothermic reaction is shown on the right. ...
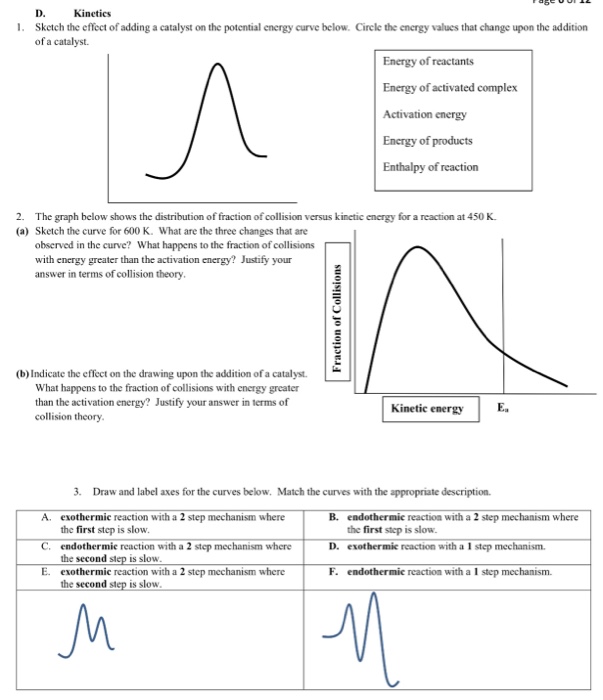
State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer--
Endothermic potential energy diagram
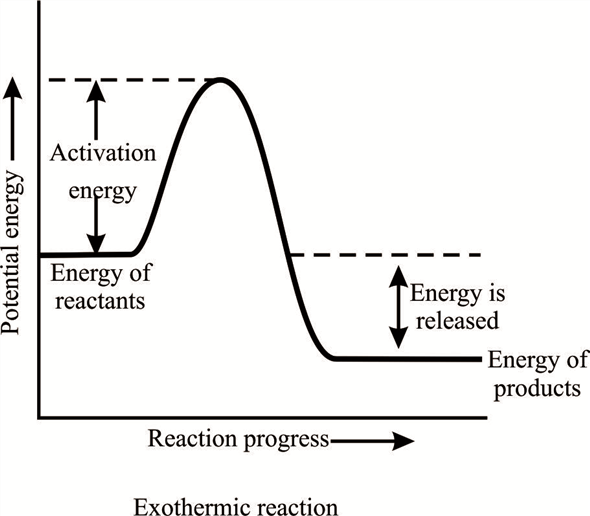
Jun 01, 2018 · Energy level diagram for an exothermic reaction is shown below. Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Potential Energy Diagram Worksheet. Adrianne Lefevre. November 15, 2021. Potential Energy Diagram Practice Endothermic And Exothermic Reactions Potential Energy Energy Activities Exothermic Reaction. Chemistry 30 Chemical Kinetics Potential Energy Diagrams Revisited Chemistry Education Teaching Chemistry Chemistry. Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
Endothermic potential energy diagram. Write a two to four sentence conclusion statement explaining how the potential energy diagram is used to identify if the reaction is endothermic or exothermic, if heat was released or absorbed, and why the sign of enthalpy change was positive of negative. There should be a conclusion statement for each graph. SINGLE REPLACEMENT GRAPH CONCLUSION: This graph is an Exothermic graph. Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ... Increase in kinetic energy - energy absorbed - endothermic Decrease in kinetic energy - energy released – exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. In the profile for an exothermic reaction, the overall change is negative. You can tell this because the products have less energy than the reactants, and the arrow showing the overall change in energy points downwards. ... The diagram shows a reaction profile for an endothermic reaction.
September 7, 2021 - The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy ... A video from Energy Foundations for High School Chemistry. Oct 7, 2015 - This Pin was discovered by Eric Tobias. Discover (and save!) your own Pins on Pinterest In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:
March 3, 2018 - Here is a potential energy diagram for an endothermic reaction. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. If the initial state has a lower potential energy than the final state, the process is endothermic. An energy diagram shows he change in energy that occurs when a given reaction takes place. It usually includes reactants, products, and transition states.
20 Dec 2015 — Why is the respiration reaction exothermic? Why is combustion an exothermic reaction? How can I read the potential energy diagrams when there is ...1 answer · http://www.everythingmaths.co.za An endothermic reaction must have a positive change in enthalpy. That is, ΔH>0. This means that the system absorbs heat. ...
Endothermic and exothermic reactionsPaul Andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically.
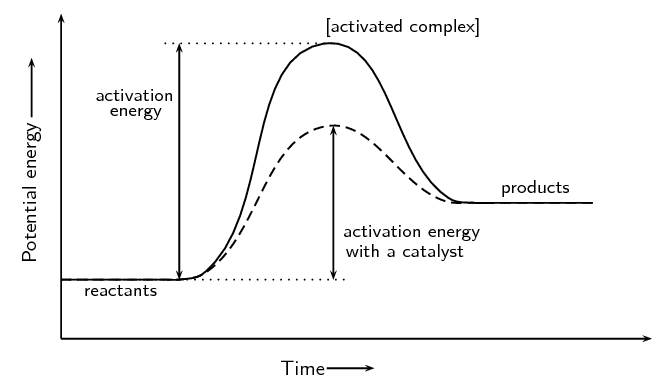
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Answer (1 of 4): And yet again, a lazy, stupid student who just pastes their homework question into Quora, not even politely asking for help and referring to a diagram that ISN'T supplied. No wonder you don't know the answer. Yes, I'm being honest, and cruel, but I hope you can see that you are...
This collection of web resources contains materials for the core units of the Saskatchewan Evergreen Curriculum for Chemistry 30. Links to each unit are found in the gray navigation bar near the top of each page · Each unit contains the following resource materials:
Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
Nov 25, 2021 · A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction.

Nuclear powerplant in Belgium Please mention me on Instagram: @Fredpaulussen or link to my website fredography.be Thank you!
This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
10+ Endothermic Energy Diagram. Endothermic reactionin an endothermic reaction, the products are higher in energy than the an energy diagram can be used to show energy movements in these reactions and temperature can be. An energy level diagram shows whether a reaction is exothermic or endothermic. Energy is absorbed δh = + (net gain).
Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the ...
9 Jul 2019 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
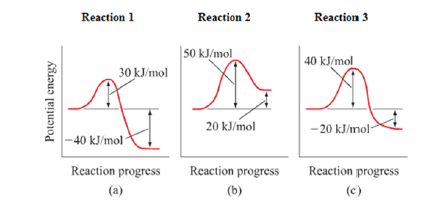
3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement. Reaction I Reaction 2 a) E P of the reactants C R b) E P of the products E Z c) ∆H of the reaction B S d) activation energy of the
Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
May 2, 2017 - It might help to draw dashed lines so that you can visualize the potential energy all the way across the graph Reactants Products Activation Energy · 9. Energy level diagram for an exothermic chemical reaction without showing the activation energy. Very endothermic reaction with a large activation ...
23 Feb 2012 — draw and label the parts of a potential energy diagram. Vocabulary. endothermic reaction; exothermic reaction; internal energy; potential energy ...
An endothermic reaction is one in which heat energy is absorbed. The products have more enthalpy than the reactants therefore \(\Delta H\) is positive. ... The activated complex (high energy intermediate state where bonds are breaking and forming) can be shown on potential energy diagrams.
In the graph for an endothermic reaction, you can see that the products have a higher potential energy, implying that energy has been put into the system. This further proves that ΔH is positive for an endothermic reaction. Image Courtesy of SilaVula Example
Dec 13, 2013 - Posts about IB Chemistry written by marvinamin
May 22, 2006 - In our unit on Thermochemistry the terms endothermic and exothermic were discussed. These are terms you likely learned in Chemistry 20 but it is important to review them: · Endothermic reactions require a net input of energy
Since heat is released for C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction.
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction ...
Potential energy 20 reacüon pathway Parts of the Potential Energy Diagram 1) Reactants: First flat line from left 2) Products: Second flat line from left 3) Heat of Reaction, AH : Hf— Hi The difference in enerw from the reactants to the products. +/XH = Endothermic -AH = Exothermic 4) Activation energy: The energy needed to go from the ...
Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
Potential Energy Diagram Worksheet. Adrianne Lefevre. November 15, 2021. Potential Energy Diagram Practice Endothermic And Exothermic Reactions Potential Energy Energy Activities Exothermic Reaction. Chemistry 30 Chemical Kinetics Potential Energy Diagrams Revisited Chemistry Education Teaching Chemistry Chemistry.
Jun 01, 2018 · Energy level diagram for an exothermic reaction is shown below. Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled.






















0 Response to "36 endothermic potential energy diagram"
Post a Comment